Article Text
Abstract
Lung function is a predictor of morbidity and mortality, and the chronic nature of lung function decline allows for preventive initiatives. Proinflammatory constituents of organic dust are considered a possible cause of compromised respiratory health. The aim of this systematic review was to reveal the impact of organic dust exposure on long-term change in lung function. The literature search was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) criteria. Predefined criteria concerned study design: longitudinal, ≥1 year follow-up, ≥50 exposed; exposure measures: organic dust, measured or estimated, in different occupational settings; and outcome measures: change in lung function measured by spirometry. Based on these criteria, 1580 potentially relevant publications were narrowed down to 20 included publications. Quality was evaluated and discussed based on six objectively defined criteria. Overall, 14 studies found some type of association between exposure to organic dust and long-term change in lung function. However, the results were inconsistent and no specific work exposure showed more clear associations to change in lung function. Meta-analysis revealed an overall small significant excess loss in forced expiratory volume in the 1st s for exposed compared with controls of 4.92 mL/year (95% CI 0.14 to 9.69). No significant association was seen overall for forced vital capacity. 12 studies revealed a significant exposure–response relation between organic dust and change in lung function. The results were inconsistent across varying study design and different exposure measures and outcomes. We therefore conclude that there is limited evidence of a causal association between general exposure to organic dust and long-term excess decline in lung function.
- Lung function change
- occupational exposure
- organic dust
- epidemiology
- review
Statistics from Altmetric.com
What this paper adds
This is the first systematic review on the association between organic dust and decline in lung function.
It shows limited evidence of a causal association between exposure to organic dust and change in lung function, with a small significant excess decline in only forced expiratory volume in the 1st s of 4.92 mL/year (95% CI 0.14 to 9.69) among exposed compared with controls.
Introduction
The definition of several chronic pulmonary diseases, such as asthma and chronic obstructive pulmonary disease, includes reference to lung function, and they are associated with accelerated lung function decline. These diseases are a leading cause of morbidity and mortality and are a global health problem with increasing prevalence, leading to a substantial burden worldwide.1 2 Lung function on its own is also a predictor of morbidity, mortality3–5 and cognitive and physical functioning6 in the general population. Change in lung function over time is therefore of interest, and because of the slow evolution and chronic nature of lung function decline it presents opportunities for prevention.7
Organic dust—an aggregate of particles from plants, animals and microbes suspended in the air,8 containing for example bacteria, moulds and pollens—is a well-established major air pollutant within different workplaces. The mechanisms of lung damage from organic dust may work partly through one of its constituents, endotoxin. Endotoxin is a building stone of the outer membrane of Gram-negative bacteria and is considered to be a possible cause of respiratory disease among workers exposed to organic dust due to its strong potency in comparison with other proinflammatory microbial constituents of organic dust.9 The association between exposure to endotoxin and acute decline in lung function was first described by Castellan et al 10. They found a strong relation between airborne endotoxin exposure and mean percentage acute decline in forced expiratory volume in the 1st s (FEV1), whereas the dust concentration per se was not correlated with an acute change in FEV1.10
Occupational exposure to organic dust and the possible association with level of lung function have been investigated in several studies for different occupations, especially cotton work, wood work, farming and grain work. There is also an increasing interest in exposures in utero and early in life and the impact this has on the developing lung.11 Cross-sectional studies, however, cannot uncover the sequence of events and is not well suited to infer causality.12 In this systematic review we therefore aimed to examine the effect of exposure to organic dust in different occupational settings on longitudinal change in lung function and to clarify where future research contributions are needed. To our knowledge no other systematic review concerning lung function change in relation to exposure to organic dust has been conducted.
Methods
Search strategy
The literature search was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) criteria, a recommended method for systematic reviews.13 The search strategy, inclusion and exclusion criteria were developed among the authors. The first author conducted the literature search identifying available literature by searching three online databases (National Center for Biotechnology Information (NCBI) PubMed, Embase and Cochrane Library) for publications prior to 1 May 2016. The full electronic search was optimised using NCBI PubMed, after which the same search was performed in the other databases. Free text searches were prioritised in each database after exploding relevant index terms (ie, Medical Subject Headings (MeSH)) to ensure all articles of interest to be found and to avoid missing articles not yet categorised in index terms. The full electronic search strategy is provided in the online supplementary table S1, and the online supplementary figure S1 shows the full example of the final search performed in NCBI PubMed. A broad search using the overall search terms ‘organic dust’ and ‘change in lung function’ was performed in order to identify studies on change in lung function and organic dust exposure. This search was supplemented with search words for occupations assessed to be exposed to organic dust based on expert judgements of the authors, including agriculture/farming, woodworkers, waste workers, compost workers, bakers, grain workers and saw mill workers (see online supplementary table S1). Identification of the relevant studies based on the described inclusion and exclusion criteria below were made and agreed on by both the first and last authors, first by title, then by abstract and finally by full article.
Study criteria
The following were the inclusion criteria: (1) design: longitudinal/prospective cohort studies with a minimum of 1 year follow-up time; (2) exposure: organic dust, endotoxin and/or other dust-associated exposures in different work settings (see search in online supplementary table S1); (3) outcome: longitudinal change in the lung function indices of forced expiratory volume in the 1st s (FEV1), forced vital capacity (FVC) and/or FEV1/FVC; (4) study subjects: humans; (5) language: English-language, full-length, original publications in peer-reviewed journals.
Studies were excluded based on (1) design: cross-sectional or cross-shift studies and follow-up time under 1 year, (2) exposure: inorganic dust exposure or ‘mixed’ dust exposure without opportunity to separate organic and inorganic dust, (3) lack of exposure gradient, such as exposed versus controls or without different levels of exposure among the exposed subjects, (4) prognostic studies of specific patient groups, (5) size: studies with <50 exposed subjects, (6) lack of a quantitative estimate of association between lung function change and organic dust exposure and (7) lack of adjustment for smoking.
Several cohorts had been followed up in different cycles and therefore described at different follow-up times (ie, Shanghai cotton textile study,14 Saskatchewan grain study15 and farmers in the Doubs province16). In order to avoid repeated cohorts inflating their effect on the review, we have reported (in tables 1 and 2) the articles that come closest to our interest, namely study aim and the greatest exposure contrast (years of exposure). Other studies concerning the same cohorts have been shortly described in the text.
Characteristics of studies included in the review
The effect of exposure to organic dust on the change in lung function over time, study results
Due to previous criticism of ad hoc-defined quality scoring as lacking validity17 and evidence that using different quality score systems is problematic,18 we decided to use our own objectively defined criteria concerning quality and hence discuss key components of design, rather than aggregate scores themselves. Consequently the quality of the studies was evaluated and discussed based on objectively defined criteria concerning study design (longitudinal=1 (all)), follow-up time (≥6 years=1), response rate (>50%=1), exposure measure (dust measurements=1), exposure–response relation (analysed=1), and confounder control (all of smoking, sex, age and height=1) (table 3). Random effects meta-analysis was performed for the outcome measures FEV1 and FVC comparing exposed versus controls (figure 2A,B). For studies where analyses were made but results stated as non-significant without described effect sizes, a coefficient of 0 and an SE of the mean of all other included studies SEs were given. For FEV1 this was done in one study19 and for FVC in two studies.20 21
Quality scores of the included studies
Results
Study selection
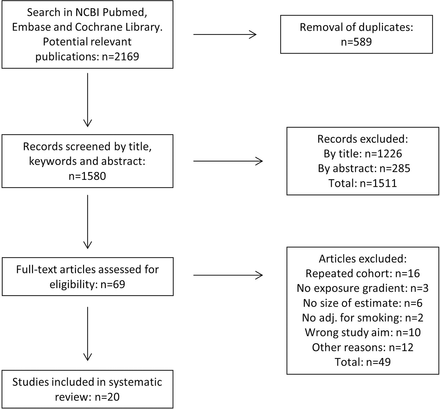
All manuscripts that met the search criteria were assessed in a common database (www.refworks.com). After removal of duplicates (n=589), there were 1580 potentially relevant publications (figure 1), which were screened based on title and abstract, leading to exclusion of 1511 studies: 1226 based on their title and 285 based on their abstract. Based on a full-text screening of the remaining 69 articles, 49 articles were excluded due to either a lack of suitability in terms of study aim, study design, size of exposed group, exposure or outcome variables, no quantitative estimate of association, or repeated cohorts (figure 1). From a snowball search in the 20 remaining publications and 8 review articles22–29 concerning organic dust and respiratory diseases, there were no additional articles that fulfilled the inclusion criteria. Therefore 20 publications were included in this review.
Flow diagram of study selection for the systematic review.
Characteristics of studies
The characteristics of the 20 included studies14–16 19–21 30–43 are summarised in table 1. All studies were prospective cohort studies with follow-up times between 1 and 20 years (median: 6.5 years). Sample sizes ranged from 97 to 11 827 exposed individuals, with a median of 218. Ten studies14 16 19 32 35–37 39 42 43 had an external control group for comparison, and ten15 20 21 30 31 33 34 38 40 41 had an internal control group within the occupation (such as swine farmers compared with dairy farmers) or compared levels of exposure within the exposed group. The mean age at baseline ranged from 18.3 to 50.8 years, and the cohorts originated from Canada,15 32 33 35 39 43 China,14 31 Denmark,19 20 36 42 France,16 37 38 The Netherlands34 40 and USA.21 30 41
The included studies encompassed the five exposure types: (1) cotton dust,14 30 31 (2) grain dust,15 32–35 (3) farm dust,16 20 21 36–40 (4) paper dust19 and (5) wood dust.41–43 In 12 studies14 19 21 30–32 34 36 40–43 the exposure was measured with personal or stationary dust collectors, with individual exposure estimates for each subject. In eight studies15 16 20 33 35 37–39 the exposure was assessed from questionnaires as time spent working in different exposure settings, or years working in the industry, or as simple dichotomous variables such as exposed versus control or yes/no to different work characteristics.
The outcome in all studies was change in lung function of one or more of the indices FEV1, FVC and FEV1/FVC measured by spirometry at a minimum of two occasions with at least 1 year in between. All studies were according to the inclusion criteria adjusted for smoking, the strongest predictor and possible confounder for change in lung function. Most studies furthermore adjusted for age, sex, height and weight, and some studies adjusted for baseline lung function19 32 33 39–41 and altitude.16 37 38 However, there were different approaches and selection criteria for included confounders across the studies.
The quality score of each study is summarised in table 3. Each element of the scores is discussed for the included articles in the Discussion section.
Findings
The results of the 20 included studies are summarised in table 2. The reported results were heterogenic. Most studies presented results of multivariable linear regression models. Overall 14 studies found a negative association between exposure and decline in lung function (FEV1, FVC and/or FEV1/FVC), either when comparing exposed subjects with controls14 16 21 35 39 42 43 and/or as exposure–response relations within an exposed population.14–16 21 30 32–34 37 40 42 43
Of the three studies on cotton workers, one study by Wang et al 14 found that cotton workers had a significant greater decline in FEV1 of 360 mL compared with silk workers over 20 years of follow-up (corresponding to an excess decline of 18 mL/year). In this study they also found an exposure–response relation between endotoxin level and loss in FEV1 (highest vs lowest level: −155.3 mL over 20 years). An exposure–response relation was also seen in the cotton study by Glindmeyer et al,30 but only among yarn manufacturing workers, who were the lowest exposed group of workers, compared with slashing and weaving. An increase of 100 µg/m3 average cotton dust exposure in yarn manufacturing workers led to a significant decline in both FEV1 (−16.2 mL/year) and FVC (−18.0 mL/year). However, in their comparison of cotton workers with controls (synthetic workers), an opposite association was seen, with the low exposed control group having the greatest decline in FEV1 (−13.14±3.13 mL/year (p<0.01)). The last study by Wang et al 31 found no association between cotton dust exposure and change in lung function during only 1 year of follow-up of newly hired female cotton workers.
Among the five studies on grain workers, an association was found between increasing time in the industry (years) and annual decline in lung function in three studies (FEV1: −4 mL/year and FVC: −6 mL/year,15 FEV1: −0.40% predicted/year and FVC: −0.34% predicted/year32 and FEV1: −0.6 mL/year per week in the industry33). One study by Post et al 34 found no significant difference in annual decline in lung function between subjects with >5 years of exposure compared with no exposure or <5 years of exposure. This study, however, found a significantly increased annual decline in FEV1 among high grain dust-exposed (−58.2 mL/year, excess decline of 22.2 mL/year) compared with low grain dust-exposed (−35.8 mL/year). This association was not seen for endotoxin exposure. One study by Senthilselvan et al 35 found that grain farmers had an excess annual decline in FVC of 9.2 mL/year compared with controls, although no significant exposure–response relation was observed between the rate of change in FVC per year and years of grain farming in this study.
Eight included studies explored the effect of farming exposure on change in lung function. Three studies16 21 39 showed a significant difference in lung function decline (FEV1: −38 mL/year,21 FEV1: −26.1 mL/year and FVC: −33.5 mL/year39 and FEV1/FVC: −0.21%/year16) for farmers compared with controls. Two studies36 37 found no significant difference for lung function change between farmers and controls. One study by Iversen and Dahl20 looked at the difference in change in lung function between pig farmers and dairy farmers and found no significant difference. A study by Mauny et al 38 compared barn-drying farmers with traditional drying farmers and found no difference in change in lung function between the groups. Exposure–response relation was found in two studies,21 40 the first showing that total endotoxin (EU/m3) was significantly associated with annual decline in FEV1 (−26 mL/year), and the second showing that an increase in endotoxin exposure with a factor 2 was significantly associated with an excess decline in FEV1 of 19.4 mL/year. FVC change was significantly associated with a factor 2 increase in both endotoxin (−40.7 mL/year) and inhalable dust exposure (−41.2 mL/year). One study from our own group by Bolund et al 36 found no relation between either dust or endotoxin exposure and change in lung function among young farmers.
A study of paper workers by Sigsgaard et al 19 showed no significant excess loss of lung function among workers exposed to up to 200 EU/m3 of endotoxin compared with controls.
Of the three included studies on wood workers, one study by Noertjojo et al 43 showed that sawmill workers had a significantly greater annual decline in FEV1 (12.1 mL/year) and FVC (14.6 mL/year) compared with controls. They also found a significant exposure–response relation between wood dust and decline in FVC, where high exposed subjects had an excess decline of 21.3 mL/year, and medium exposed subjects 15.8 mL/year compared with the lowest exposed. A study by Glindmeyer et al 41 showed no association between wood dust exposure and adverse effects on lung function. However, in a subanalysis they found an exposure–response relation between respirable residual particulate matter and decline in FEV1 and FVC. The last study by Jacobsen et al 42 found a significant exposure–response relation between cumulated wood dust exposure (mg/m3/year) and decline in FEV1 and FVC for female workers (FEV1: −3.1 mL/mg/m3/year, FVC: −3.0 mL/mg/m3/year). This was not seen among males.
A few studies explored effect modification by sex and/or smoking.36 39 42 43 Two studies34 42 found evidence of effect modification by sex; one study on farmers36 found that the negative effect on lung function from being a current farmer compared with an ex-farmer was larger for females than for males; the other study on wood workers42 showed that female wood workers with the highest exposure had a significant excess decline in FEV1 (25 mL) compared with the lowest exposure, which was not seen for males. Of those studies that explored effect modification by smoking,36 39 43 no studies found clear indications of significant interaction between smoking and exposure on the decline in lung function.
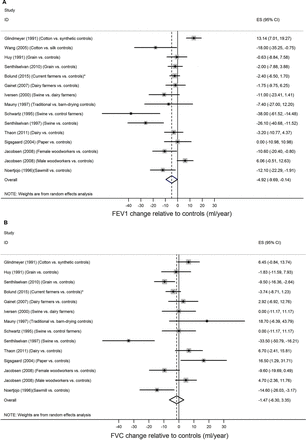
Meta-analysis of the effect of dust exposure on FEV1 for exposed compared with controls in 14 studies revealed an overall excess loss of 4.92 mL/year (95% CI 0.14 to 9.69) (figure 2A). No excess decline was found in the meta-analysis for FVC (figure 2B) (excess decline in FVC: 1.47 mL/year (95% CI −3.35 to 6.30)).
(A) Meta-analyses of the effect on FEV1 among exposed versus controls (or other working group with lower exposure). (B) Meta-analyses of the effect on FVC among exposed versus controls (or other working group with lower exposure). FEV1, forced expiratory volume in the 1st s; FVC, forced vital capacity.
Discussion
This is, to our knowledge, the first systematic review on long-term change in lung function among populations exposed to organic dust. Overall we found heterogeneous results; however, meta-analyses pointed towards an overall small significant effect of organic dust exposure on FEV1 decline. This was not seen for FVC. Overall 14 studies14–16 21 30 32–35 37 39 40 42 43 out of 20 found a significant association between exposure to organic dust and change in one or more of the lung function indices (FEV1, FVC and FEV1/FVC). The remaining six studies19 20 31 36 38 41 found no significant change in lung function in relation to organic dust exposure.
For all types of exposure (cotton, grain, farming and wood dust), inconsistent results were reported for the association between exposure and change in lung function. Therefore it is difficult to conclude if any specific exposure type was more evident to be associated with decline in lung function. Grain dust exposure was the most consistent, with all five studies finding some type of association with any of the three lung function indices, but not all grain studies showed a significant exposure–response relation. Furthermore, the results do not consistently point towards either endotoxin or dust per se as a risk factor for change in lung function. One study showed a significant association for dust but not endotoxin,34 whereas endotoxin in other studies was the significant risk factor for excess lung function decline.14 21 However, the two exposures tend to be strongly correlated, and can hence be difficult to assess separately.
The units of exposure used to assess exposure–response relations between dust/endotoxin and change in lung function varied between studies, which makes it challenging to compare effect size of exposure–response relations across studies. Random effects meta-analysis, for studies comparing exposed with controls, revealed a small overall effect of dust exposure on FEV1, but not on FVC (figure 2A,B). Studies exploring only exposure–response relations were not included in these meta-analyses. Therefore, it must be kept in mind that the meta-analyses do not fully cover all the associations found in this review. Furthermore, the choice of control group may be very influential for the associations found. Some studies used an external control group thought to be exposed to no organic dust; other studies included control groups within similar occupations but with known lower dust exposures. In these studies, both the level and possibly also the dust potency may influence the associations found.
Quality scores for each included study were based on our own objectively defined criteria (table 3). All studies were longitudinal and had to be adjusted for smoking as fulfilment of the inclusion criteria. Follow-up time is of great importance, and in order to reliably assess lung function decline, several years of observation is required to achieve robust estimates, as argued by Burrows et al in a study on changes in the normal flow–volume curve with growth and ageing,44 and by an official statement from the American Thoracic Society on spirometry in the occupational setting.45 Therefore we did an assessment of the results restricted to the 11 studies with follow-up time of ≥6 years,14–16 19 20 32 35–37 42 43 but this restriction revealed similar inconsistent results of the association between exposure and change in lung function. The response rate in the studies varied from 30% to >85%, so even with a cut-off at 50% to define a satisfactory response, there is a risk of selection bias due to differences between responders and non-responders in all studies. We would expect that non-responders are the most vulnerable subjects, and this would lead to the risk of underestimating the true effect. However, both in low and high response studies, the reported associations were inconsistent. Most studies (18/20) explored exposure–response relations as change in lung function in association with either increasing dust/endotoxin level or with increasing exposure time. Twelve studies14 19 21 30–32 34 36 40–43 reported individual exposure estimates for each subject based on personal or stationary dust collectors. These criteria, which help evaluate the possible effect with greater precision, did not show different effect sizes between groups. Confounder control included smoking (inclusion criteria), sex (adjustment, stratification or restriction to one sex), age and height. It can be discussed if height adjustment should be included as a confounder in longitudinal analyses of lung function in adult populations. However, we decided to include height adjustment as a quality criterion, as it is of importance to the young subjects in the cohorts that may not be fully grown, and hence possibly for the overall result.
Strengths and limitations
The strengths of this review include that all of the 20 included studies were longitudinal studies with a minimum of 1 year of follow-up and with information on smoking. Each cohort was only included once in order to contribute equally to the results. Because of the known detrimental effect of smoking on lung function, all included studies had to take smoking into account, and we believe that the thorough adjustment/stratification for smoking in all included articles makes it less likely that the results are biased by smoking. Other confounders considered were sex, height, age, altitude, weight or weight gain, different symptoms, follow-up time and baseline lung function. The approach of confounder selection was heterogenic between studies. It is debated whether or not to adjust for baseline lung function when assessing change in lung function over time. Adjusting for the baseline level of lung function is problematic because of correlated errors between lung function level and lung function change.46 The effects of these correlated errors on the regression coefficients may lead to bias due to the ‘horse-racing effect’,47 48 whereby loss is negatively associated with attained level.46 Results adjusted for baseline lung function may therefore be biased, but as several studies chose this approach19 32 33 39–41 43 we chose to include them. Studies with and without adjustment for baseline lung function did not differ systematically in terms of the associations found.
Limitations include the fact that the studies chose different approaches of analyses, focus on different lung function indices (FEV1, FVC and FEV1/FVC), and differed in how the change in lung function was reported and how the exposure was assessed. Reporting bias may also be of concern due to the known risk of publication of studies with significant results, as well as selective reporting by the authors of the respective articles, choosing to emphasise on significant results rather than negative results. In this review we were not able to assess to what extent reporting bias may be a problem, because all included studies report on several outcomes (FEV1, FVC and FEV1/FVC), whereof some outcomes were associated to the exposure, whereas others were not.
Lung function change over time was in most studies presented as absolute change or, in some studies, as change relative to baseline. One study reported change in per cent predicted.32 Only one study,36 from our group, used z-scores from the GLI2012 equations.49 Age is an important factor for change in lung function, as lung function decline above the age of 30 is evident50 51; moreover, the variability of individual measurements around the median is not uniform across all ages and heights.52 It has been argued that conventional multiple regression analysis is not adequate to model the complex relationship between body size, age and lung function.52 The GLI2012 equations account for the effect of ageing, height, sex and ethnicity, and should be the most precise way to assess lung function across all ages. A z-score approach may also be more meaningful when evaluating FEV1 over time, as shown in a new study by Vaz Fragoso et al,53 as FEV1 assessed as z-scores was shown to be more frequently associated with multiple cardiopulmonary predictors than measures of FEV1 in litres, as % of predicted and as L/m3.
According to the followed PRISMA criteria,13 two assessors needed to agree on inclusion and exclusion of articles after an initial independent selection. The majority of articles were excluded based on screening of titles (n=1226) and abstracts (n=285) out of the initially identified (n=1580), which may have led to some articles of potential relevance for the study to have been excluded. However, we believe that the procedure with two assessors limits this risk, and that the possible false discarding of one or more articles should not influence the result of the study or bias our overall conclusions of the review.
Articles concerning repeated cohorts
Several cohorts were examined repeatedly during their follow-up time, and therefore several articles were published concerning the same cohorts. The Shanghai textile worker study had six cycles with 5-year intervals during 1981 to 2011, leading to several results.54–61 They reported both no exposure–response relation but significant differences between cotton workers and silk workers,58 and significant exposure–response relation for both endotoxin levels14 56 as the risk factor for negative change in lung function, and at another follow-up time dust level as a risk factor.55 The Shanghai textile worker study also studied lung function improvement after exposure cessation and found an FEV1 improvement after cessation of cotton and silk work,59 61 and identified prior occupational exposure levels and sex as important modifiers of FEV1 recovery.61 The Shanghai study also explored genotypes associated with enzyme activity,62 and found that genotypes associated with ‘slow’ enzyme activity led to inefficient metabolising of reactive oxygen species generated by endotoxin exposure, and this may eventually induce faster lung function decline. These findings open doors for future research for understanding the biological mechanisms of this issue, but it is out of the scope of this review.
The farmers cohort from 1986 in the Doubs province, France, also reported results at different follow-up cycles. At 6 years of follow-up,63 lung function of farmers had deteriorated slightly more rapidly than that of the control subjects. This was not seen at 12 years of follow-up,37 where the association was non-significant when comparing farmers with controls, although the mean duration of exposure was significantly associated with the decline in FEV1. Another cohort from 1994 of farmers in the Doubs province showed that dairy farming was associated with an accelerated decline in FEV1/VC (vital capacity) per year but not VC/year or FEV1/year after a 5-year follow-up64 compared with controls, and that the modernisation of the farm had a beneficial impact on lung function. After 13 years of follow-up,16 a greater decline in FEV1/FVC ratio remained for dairy farmers.
Grain workers in Saskatchewan, Canada, showed consistent results of annual loss of lung function to increase with the number of years in the grain industry both at 6 years65 and 15 years of follow-up,66 and also was able to measure a beneficial effect on lung function from implementing dust control.15 66
Biological plausibility and causality
A negative effect of organic dust on change in lung function is biologically plausible. After inhalation and deposition of organic dust and its constituents in the airways, the particles may interact with immune cells. A strong inflammatory response may lead to lung function decline in exposed subjects. The individual immunological response to dust and endotoxins is determined by the complex interaction between dose and timing of exposure, other environmental factors and genetic predisposition.67 The mechanisms that link the immunological response in the lung with possible accelerated loss of lung function are still not fully understood and may also involve factors related to genetics, immune regulation and mechanisms related to cellular repair and the resolution of inflammation, as hypothesised in the review by Omland et al.29
Although the studies are longitudinal, it cannot be certain that the outcome occurs alongside the exposure because the lung function decline could have occurred in the beginning of the follow-up period, prior to the exposure assessed at follow-up. One way to cope with this is to include repeated measurements of lung function and exposure during follow-up. Several studies have repeated cycles, but analyses were generally made at each follow-up time and not modelled for change in exposure and change in lung function during the full follow-up.
The mean age at baseline in the included studies ranged from 18 to 51 years. It could be hypothesised that the effect of the exposure may differ according to age of the subjects, but the results of this review do not support this. Furthermore, the risk of healthy worker selection bias, which is a challenge most longitudinal studies have to deal with, may be greater when the cohort is older and the possible years of exposure greater. A study by Zejda et al 68 showed that the average decline in lung function over the first year with exposure to grain dust was associated with the subsequent exposure duration, that is, a smaller decline in lung function on the first year of exposure predicted a longer subsequent exposure duration. They concluded that the restriction of analyses to the ‘survivors’ may underestimate the relation between the exposure and the respiratory impairment. This could be an evident problem in all the studies included in this review and may even suggest that the associations found are underestimated.
The strength of the associations found in most of the studies in this review lay close to the border of significance (p<0.05). The size of the effect may, however, sum up to be of importance to the individual, as an excess decline in FEV1 of 5 mL/year could lead to possible important health issues after many years of exposure. Furthermore, higher exposed subjects may be at greater risk of excess decline in lung function than lower exposed subjects if the exposure–response relations are true. The results in this review were, however, not consistent, but 1414–16 21 30 32–35 37 39 40 42 43 out of 20 studies found results that point towards a negative effect of organic dust exposure on lung function change.
The findings of this review illustrate the complexity of assessing change in lung function in occupational settings. Focus on possible adverse health effects associated with occupational exposure to organic dust should continue, as well as protection of the individual working in occupations with high dust exposure. Future directions within the field should be to continue with well-powered follow-up studies, acquire precise exposure measures and take healthy worker selection bias into consideration (ie, follow-up young cohorts and examine both active workers and dropouts).
Conclusion
The studies included in this review were of varying design, applied different measures of exposure and outcome, and had varying follow-up times. Meta-analysis revealed an overall small significant excess loss in FEV1 for exposed compared with controls of 4.92 mL/year (95% CI 0.14 to 9.69). However, the results were inconsistent, and we therefore conclude that there is only limited evidence of a causal association between exposure to organic dust and long-term excess decline in lung function.
References
Footnotes
Contributors ACSB, corresponding author, has contributed to the literature search, the identification of relevant articles, full-text screening, drafting the article and final approval of the version to be published.
VS has contributed to the identification of relevant articles, drafting the article, revising it critically for important intellectual content and final approval of the version to be published.
TS and MRM have contributed to revising the article critically for important intellectual content and final approval of the version to be published.
ACSB and VS are responsible for the overall content as guarantor(s).
Funding Funding of the present work was supported by the University of Aarhus, Graduate School of Health.
Disclaimer The authors declare no conflicts of interest, have no relevant affiliations or financial involvement associated with the subject matter or materials discussed in the manuscript.
Competing interests None declared.
Patient consent Review of previous studies.
Provenance and peer review Not commissioned; externally peer reviewed.