-
PDF
- Split View
-
Views
-
Cite
Cite
Russel J. Reiter, Dun Xian Tan, Ahmet Korkmaz, Sergio A. Rosales-Corral, Melatonin and stable circadian rhythms optimize maternal, placental and fetal physiology, Human Reproduction Update, Volume 20, Issue 2, March/April 2014, Pages 293–307, https://doi.org/10.1093/humupd/dmt054
Close - Share Icon Share
Abstract
Research within the last decade has shown melatonin to have previously-unsuspected beneficial actions on the peripheral reproductive organs. Likewise, numerous investigations have documented that stable circadian rhythms are also helpful in maintaining reproductive health. The relationship of melatonin and circadian rhythmicity to maternal and fetal health is summarized in this review.
Databases were searched for the related published English literature up to 15 May 2013. The search terms used in various combinations included melatonin, circadian rhythms, biological clock, suprachiasmatic nucleus, ovary, pregnancy, uterus, placenta, fetus, pre-eclampsia, intrauterine growth restriction, ischemia-reperfusion, chronodisruption, antioxidants, oxidative stress and free radicals. The results of the studies uncovered are summarized herein.
Both melatonin and circadian rhythms impact reproduction, especially during pregnancy. Melatonin is a multifaceted molecule with direct free radical scavenging and indirect antioxidant activities. Melatonin is produced in both the ovary and in the placenta where it protects against molecular mutilation and cellular dysfunction arising from oxidative/nitrosative stress. The placenta, in particular, is often a site of excessive free radical generation due to less than optimal adhesion to the uterine wall, which leads to either persistent hypoxia or intermittent hypoxia and reoxygenation, processes that cause massive free radical generation and organ dysfunction. This may contribute to pre-eclampsia and other disorders which often complicate pregnancy. Melatonin has ameliorated free radical damage to the placenta and to the fetus in experiments using non-human mammals. Likewise, the maintenance of a regular maternal light/dark and sleep/wake cycle is important to stabilize circadian rhythms generated by the maternal central circadian pacemaker, the suprachiasmatic nuclei. Optimal circadian rhythmicity in the mother is important since her circadian clock, either directly or indirectly via the melatonin rhythm, programs the developing master oscillator of the fetus. Experimental studies have shown that disturbed maternal circadian rhythms, referred to as chronodisruption, and perturbed melatonin cycles have negative consequences for the maturing fetal oscillators, which may lead to psychological and behavioral problems in the newborn. To optimize regular circadian rhythms and prevent disturbances of the melatonin cycle during pregnancy, shift work and bright light exposure at night should be avoided, especially during the last trimester of pregnancy. Finally, melatonin synergizes with oxytocin to promote delivery of the fetus. Since blood melatonin levels are normally highest during the dark period, the propensity of childbirth to occur at night may relate to the high levels of melatonin at this time which work in concert with oxytocin to enhance the strength of uterine contractions.
A number of conclusions naturally evolve from the data summarized in this review: (i) melatonin, of both pineal and placental origin, has essential functions in fetal maturation and placenta/uterine homeostasis; (ii) circadian clock genes, which are components of all cells including those in the peripheral reproductive organs, have important roles in reproductive and organismal (fetal and maternal) physiology; (iii) due to the potent antioxidant actions of melatonin, coupled with its virtual absence of toxicity, this indoleamine may have utility in the treatment of pre-eclampsia, intrauterine growth restriction, placental and fetal ischemia/reperfusion, etc. (iv) the propensity for parturition to occur at night may relate to the synergism between the nocturnal increase in melatonin and oxytocin.
Introduction
Even before the discovery of melatonin in the pineal gland (Lerner et al., 1958), the organ was experimentally linked to reproduction, but the findings were unpersuasive (Quay, 1956; Mogler, 1958). The decade of the 1960s, however, was accompanied by a series of seminal studies which showed that the gland functioned as a photoneuroendocrine transducer (Quay, 1963; Axelrod et al., 1964, 1965) and it was definitively proved to have a physiological association with the hypothalamo-pituitary-gonadal axis (Hoffman and Reiter, 1965, 1966; Reiter and Hester, 1966). In particular, the daily changes in the duration of darkness under natural photoperiod conditions were found, via the pineal gland, to drive seasonal variations in reproductive capability in photosensitive species (Reiter, 1973, 1974). That melatonin was the mediator of pineal origin which determined the waxing and waning of reproductive competence under these conditions was uncovered shortly thereafter (Reiter et al., 1976; Tamarkin et al., 1976; Carter and Goldman, 1983).
In addition to its importance in intervening between the changing light:dark cycle and seasonal reproduction in photoperiodic species (Revel et al., 2009; Lincoln and Hazlerigg, 2010), melatonin has numerous other actions on the gonads and adnexae of non-seasonally breeding mammals, including the human, that are beneficial to optimal peripheral reproductive organ health (Reiter et al., 2009c, 2013a). The new data relating melatonin to pregnancy and delivery are summarized in this survey.
Research on circadian rhythms progressed concurrent with that of melatonin. Given that a normal light:dark cycle is of 24 h duration and has been for eons, it was advantageous for animals to modulate their metabolism, locomotor activity, etc. over a 24-h period to preserve energy, reduce predation pressure and avoid the likelihood of antagonistic cellular processes, e.g. the maximal activities of lipolytic and lipogenic enzymes in hepatocytes, from occurring simultaneously (Li et al., 2012; Thut et al., 2012). Thus, vertebrate species developed a light-sensitive biological clock whose function is governed by the prevailing light:dark cycle, the most regular recurring variable in the environment (Brainard et al., 1983, 1984; Meng et al., 2011; Ruger et al., 2013). This master clock is located in the suprachiasmatic nucleus (SCN) of the hypothalamus (Swanson and Cowan, 1975; Lydic et al., 1980). The SCN synchronizes many circadian rhythms in humans as in other vertebrates (Gillette and Tischkau, 1999; Hofman, 2000). Since the circadian production of melatonin in the pineal gland is also under the influence of the SCN, it is often difficult to separate the biological effects of general circadian disruption from those following from disturbances in the circadian melatonin cycle (Reiter et al., 2009b, 2012b).
In the following survey, the importance of the circadian clock to optimal reproductive physiology is often discussed separately from that due to alterations in melatonin (and vice versa). In reality, however, there is often no way of knowing whether a particular abnormal process relates to a derangement of the biological clock or to a perturbation or total suppression of the cyclic production of melatonin (Dumont et al., 2012).
Methods
The published literature utilized to compile this review was retrieved from a number of sources. Because of the relative diversity of subjects reviewed (i.e. mechanisms of melatonin synthesis in the pineal and in the placenta, peripheral melatonin receptors, free radical biology and the role of melatonin and its derivatives as free radical scavengers and as antioxidants, functional aspects of the central and peripheral circadian clocks, maturation and development of fetal physiology, diseases of pregnancy and the control of parturition), search terms were broad and numerous. These included, in various combinations, melatonin, circadian rhythms, biological clock, SCN, ovary, pregnancy, uterus, placenta, fetus, pre-eclampsia, intrauterine growth restriction, ischemia/reperfusion, chronodisruption, antioxidants oxidative stress and free radicals. All germane literature uncovered is summarized in this report.
Melatonin as a free radical scavenger and as an antioxidant
Whereas an extensive discussion of the actions of melatonin as a direct free radical scavenger (Tan et al., 1993; Galano et al., 2011) and as an indirect antioxidant (Barlow-Walden et al., 1995; Rodriguez et al., 2004) is beyond the scope of this review, the data overwhelmingly show that this indoleamine is highly effective in reducing oxidative stress throughout the body (Maldonado et al., 2007; de Matos et al., 2012; Tamura et al., 2013) including in the ovary, placenta, fetus and mother (see below). In studies where melatonin was compared with other better-known antioxidants (e.g. vitamins C, E, etc.) in terms of their protective efficiency against the damaging actions of toxic oxygen and nitrogen-based reactants, melatonin has proved more effective (Martin et al., 2000a; Reiter et al., 2009a; Milczarek et al., 2010). Recently, investigators also found that when melatonin was compared with chemically designed mitochondria-targeted antioxidants, again melatonin was superior in reducing molecular damage resulting from free radicals (Lowes et al., 2013).
There are several features that make melatonin highly efficient in protecting beleaguered macromolecules from oxidative/nitrosative stress (Fig. 1). First, there are no morphophysiological barriers to melatonin, e.g. it readily crosses the blood–brain barrier and the placenta (Schenker et al., 1998; Okatani et al., 1999), and so in addition to all maternal organs it also protects the placenta and the fetus. Melatonin readily gains access to the major sites of free radical generation, e.g. mitochondria. Besides being located in several subcellular compartments (Menendez-Pelaez and Reiter, 1993; Venegas et al., 2012), melatonin actually may be produced in the mitochondria (Tan et al., 2013) where free radical generation is especially high. Not only melatonin but also several of its metabolites that are formed when it functions as a direct free radical scavenger, i.e. cyclic 3-hydroxymelatonin, N1-acetyl-N2-formyl-5-methoxykynuramine (AFMK), N1-acetyl-5-methoxykynuramine (AMK), etc. are also radical scavengers (Tan et al., 2007; Hardeland et al., 2009; Hardeland, 2012) and some may be better at doing so than melatonin itself (Galano et al., 2013). Thus, melatonin is the progenitor of a variety of free radical scavengers which function in an antioxidant cascade to prevent radical-mediated damage (Fig. 2) (Tan et al., 2002). Finally, in terms of its indirect functions in limiting oxidative stress, melatonin stimulates several antioxidative enzymes (Barlow-Walden et al., 1995; Pablos et al., 1998; Rodriguez et al., 2004), likely via receptor-mediated processes (Tomas-Zapico and Coto-Montes, 2005). Its ubiquitous distribution, ease of transfer between subcellular compartments and broad spectrum capabilities make melatonin highly effective in combatting free radical damage in the reproductive system and elsewhere.
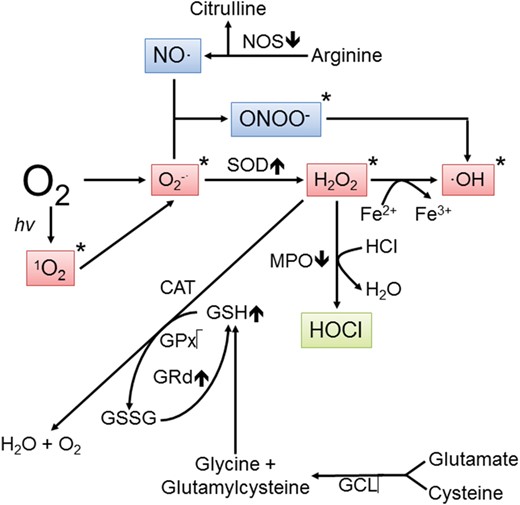
Pathways showing the conversion of molecular oxygen to reactive oxygen and nitrogen species. An estimated 1–4% of the O2 inhaled is eventually converted to reactive products. When produced in excess, free radicals and other derivatives of oxygen are highly destructive to peripheral reproductive tissues. A single electron reduction of melatonin generates the superoxide anion radical (O2•–) which is either quickly metabolized by superoxide dismutase (SOD) to hydrogen peroxide (H2O2) or it couples with nitric oxide (NO•) to produce the peroxynitrite anion (ONOO–). H2O2 is transformed to the hydroxyl radical (•OH) in the presence of transition metals, represented here by iron. O2 can also be converted to singlet oxygen (1O2), a less common but toxic species. O2•–, 1O2, H2O2 and •OH are often classified as reactive oxygen species (ROS), whereas NO• and ONOO– are classified as reactive nitrogen species (RNS). Of these products, the •OH and ONOO– are considered the most reactive and damaging. The items marked with an asterisk are scavenged by melatonin and/or its metabolites. The up and down arrows associated with the enzymes indicate whether the action of melatonin is either stimulatory or inhibitory to its activity, respectively. H2O2 is metabolized via several routes which eventually leads to the production of non-toxic species. CAT, catalase; GCL, glutamyl cysteine ligase; GPx, glutathione peroxidase; GRd, glutathione reductase; GSSG, glutathione disulfide; HOCl, hypochlorous acid; MPO, myeloperoxidase; NOS, nitric oxide synthase.
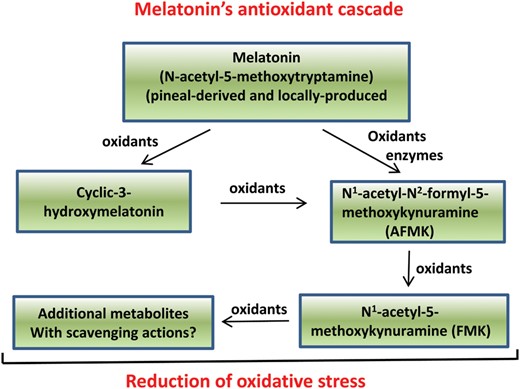
In what is referred to as the antioxidant cascade, melatonin and its metabolites function in the reduction of oxidative stress. Melatonin is a powerful antioxidant due to its ability to neutralize toxic-free radicals in both the mother and the fetus. The metabolism of melatonin is a continuum when it functions as a radical scavenger such that each metabolite, like melatonin, also detoxifies radical species. This pathway is referred to as melatonin's antioxidant cascade. In this process, not only melatonin—but also cyclic 3-hydroxymelatonin (cyclic-3OH-melatonin), N1-acetyl-N2-formyl-5-methoxykynuramine (known as AFMK) and N1-acetyl-5-methoxykynuramine (known as AMK)—all function as radical scavengers. Finally, preliminary findings indicate that the molecule(s) produced when AMK neutralizes radicals may also function as a scavenger(s). Among the metabolic products listed, cyclic-3OH-melatonin and AMK may actually be more effective radical scavengers than melatonin itself. Thus, melatonin could be referred to as a pro-antioxidant or pro-drug.
In addition to removing toxic oxygen and nitrogen species from cells by either directly quenching them or by metabolizing them to non-reactive products, melatonin also exhibits significant anti-inflammatory activity (Carrillo-Vico et al., 2005; Mauriz et al., 2013). Melatonin's efficacy as an anti-inflammatory agent stems from its ability to reduce gene expression and activities of inducible nitric oxide synthase and cyclo-oxygenase, and by limiting the production of a variety of pro-inflammatory molecules including prostanoids, leukotrienes, cytokines and adhesion molecules. Melatonin modulates the production of these factors by regulating several transcription factors including nuclear factor -κB, nuclear factor erythroid-2 related factor 2 and hypoxia-inducible factor (Mauriz et al., 2013). As a component of this process, melatonin reduces the recruitment of leukocytes to areas of injury; this also limits oxidative damage since leukocytes can be a major source of free radicals and, therefore, of the associated molecular damage.
While melatonin's multi-faceted antioxidant actions are of fundamental importance and were the presumed original functions of melatonin during evolution (Tan et al., 2013), it is not the exclusive role of melatonin to quench and/or neutralize free radicals (Reiter et al., 2010a; 2012a). Rather, this ubiquitously-acting molecule has a variety of other functions that have been well documented in mammals including man; some of these may involve not only changes in the absolute levels of melatonin but also perturbations of its circadian rhythm. Thus, changes in melatonin have been linked to sleep disturbances (Santhi et al., 2012), psychological depression (Cardinali et al., 2012), metabolism leading to body weight regulation (Fenn et al., 2011), attention deficit/hyperactivity disorder (Chaste et al., 2011) and many others. Research on melatonin and circadian rhythms is progressing at a rapid pace and it seems likely that the discoveries to date are only a fraction of the real functions of melatonin and circadian rhythmicity. How all these facets of melatonin and circadian rhythms impact reproductive physiology are only beginning to be uncovered.
Melatonin production and function in the placenta
Melatonin, N-acetyl-5-methoxytryptamine, is synthesized from tryptophan (Fig. 3) and was initially thought to be solely of pineal origin (Lerner et al., 1958). This idea was dispelled, however, when melatonin showed up in other organs along with the enzymes that produce it (Reiter et al., 2013b). While the pineal gland is the only organ in which melatonin has been found to be generated in a light:dark-dependent circadian manner (Axelrod et al., 1965; Panke et al., 1979), as reflected in the blood levels of this indole (Vaughan et al., 1976; Wehr, 1991; Kennaway et al., 1992), it is now clear that many, perhaps all, organs have acquired the ability to generate this important molecule. If melatonin is synthesized in mitochondria, as recently proposed (Tan et al., 2013), then its ubiquitous production in organisms would be assured since all eukaryotic cells are endowed with these energy-generating organelles. The advantage of mitochondria being a source of this antioxidant would certainly be fortuitous since these organelles are a major site of origin of damaging oxygen derivatives. Thus in this location, melatonin, due to its potent radical scavenging activity (Tan et al., 1993; Allegra et al., 2003; Reiter et al., 2009a; Hardeland, 2012), would be ideally situated to prevent these destructive agents from mutilating neighboring molecules. In essence, melatonin would provide on-site protection. Certainly, this is consistent with the high levels of melatonin in mitochondria (Venegas et al., 2012) and the ability of melatonin to protect this organelle from damage and preserve its physiology (Martin et al., 2000b; Acuna-Castroviejo et al., 2002, 2011; Lowes et al., 2013). The vastly different levels of melatonin in fluids and subcellular organelles have led to a debate about what constitutes a physiological concentration of melatonin (Reiter and Tan, 2003).
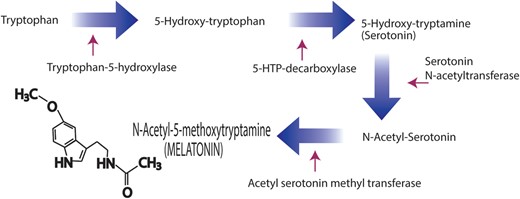
Conversion of the amino acid, tryptophan, to the indoleamine, melatonin, as documented in the pineal gland. This pathway is also utilized by a large number of other cells, including some in the peripheral reproductive system, to generate melatonin that is used locally to protect the cells from toxic-free radicals, among other functions. The villous cytotrophoblasts and the syncytiotrophoblasts from the human placenta also contain the two enzymes that metabolize serotonin to melatonin, i.e. serotonin N-acetyltransferase and N-acetylserotonin methyltransferase (also referred to as hydroxylindole-O-methyltransferase).
Specifically in the reproductive system, melatonin is reportedly produced in the ovary (Itoh et al., 1999), in the oocyte per se (Sakaguchi et al., 2013), in the cumulus cells surrounding the oocyte (El-Raey et al., 2011) and in the placenta (Lanoix et al., 2008). Moreover, melatonin levels are higher in the ovarian follicular fluid than they are in the general circulation (Brzezinski et al., 1987; Röunberg et al., 1990; Nakamura et al., 2003). In the follicular fluid, melatonin has access to the surrounding tissues where it may have the important function of protecting the oocyte from oxidative damage during both maturation and ovulation (Tamura et al., 2013), a time when free radical formation is elevated (Brannstrom and Norman, 1993; Behrman et al., 2001; Sugino, 2005). This idea is consistent with the observation that melatonin was effective in reducing molecular damage to human ova (Tamura et al., 2008a, b) that were used for IVF-embryo transfer. Just as melatonin prevents free radical damage to ova, it also does so for sperm (Ortiz et al., 2011; Reiter et al., 2013a).
The placental villous trophoblasts are not only a source of melatonin but also they contain the classic transmembrane receptors for the indole, i.e. MT1 and MT2 (Lanoix et al., 2008). To document this, villous cytotrophoblasts were isolated from human term placentas (37–41 weeks of gestation) after vaginal delivery. The two enzymes that convert the precursor, serotonin, to melatonin, i.e. arylalkylamine N-acetyltransferase (AANAT) and acetylserotonin methyltransferase (ASMT) (formerly known as hydroxyindole-O-methyltransferase) (Fig. 3) are expressed and are active in both cytotrophoblast and syncytiotrophoblast cells as well as in JEG-3 and BeWo choriocarcinoma cells, commonly used as in vitro models for human trophoblasts. Given that villous trophoblasts apparently have the capability of generating melatonin and they also possess membrane receptors, Lanoix et al. (2008) speculated that locally produced melatonin likely has paracrine, autocrine and/or intracrine actions in the placenta. In addition to working via MT1 and MT2 receptors, melatonin also could directly scavenge radicals and reduce oxidative damage to placental tissues, which would be important given the large number of radicals that are often produced in this tissue when its function becomes compromised (Pringle et al., 2010).
In the placenta, the primary villous trophoblasts include two distinct categories of cells, i.e. the mononuclear villous cytotrophoblasts (vCTB) and the multinucleated syncytiotrophoblast (STB). The fusion of the vCTB to form the STB is a highly regulated process. To ensure the transition of vCTB to the STB, the vCTB cells are generally not lost due to apoptosis. Based on the recent in vitro findings of Lanoix et al. (2012b), melatonin reduces the loss of human vCTB cells by seemingly increasing their resistance to apoptosis via the intrinsic apoptotic pathway. The suppression of the apoptotic response by melatonin in vCTB cells was shown to be a membrane receptor-mediated process, given that the addition of luzindole, an MT1/MT2 receptor antagonist, to the culture medium blocked the anti-apoptotic actions of melatonin in vCTB cells.
The STB is a non-proliferative tissue with a pro-apoptotic phenotype (Vaillancourt et al., 2009). Thus, throughout pregnancy, lost STB must be continually regenerated by fusion of vCTB cells. In the absence of the persistent input of vCTB cells into the STB, the latter would quickly be reduced in size since its life span is short (Huppertz and Kingdom, 2004; Vaillancourt et al., 2009). Because melatonin preserves the vCTB and thereby makes them available for formation of the STB, melatonin may have an important role in maintaining homeostasis in the placenta (Fig. 4). STB degeneration is a consequence of syncytial loss due to both the intrinsic and extrinsic pathways (Heazell et al., 2008). The specific actions of melatonin on the apoptotic processes of STB has yet to be defined; this action could be the same or different than melatonin's function at the level of the vCTB.
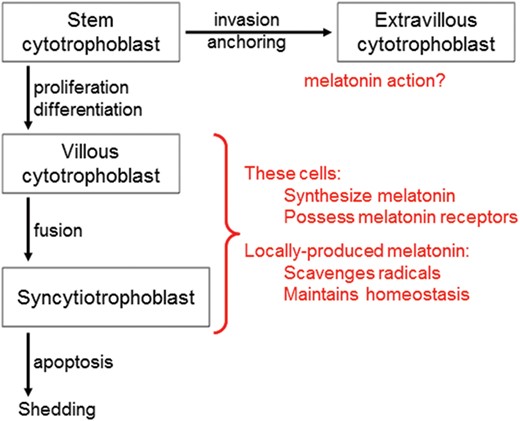
The functions of locally produced melotonin in the villous cytotrophoblasts of the placenta. The enzymatic machinery required for the synthesis of melatonin has been identified in the human villous cytotrophoblasts and in the syncytiotrophoblasts. Although clock genes have been identified in the placenta, whether there is a 24-h rhythm in melatonin production in this tissue is yet to be examined. The classic membrane receptors, MT1 and MT2, for this indoleamine are also present in the placenta. Locally generated melatonin likely functions in the protection of the placenta from oxidative stress employing both receptor-dependent and receptor-independent processes. Additionally, melatonin probably reduces the loss of villous cytotrophoblasts by preventing apoptosis of these cells; melatonin's anti-apoptotic actions are well known in normal cells. Via its influence on cell survival, melatonin presumably maintains the stable turnover of the syncytiotrophoblast. Whether melatonin has any actions at the level of the extravillous trophoblasts, especially during invasion of the uterine wall should be examined.
Interestingly, trophoblast-derived cancer cells respond differently to melatonin than do the vCTB cells. Several choriocarcinoma cell lines have been established. One of the best studied is the BeWo line (Lewis et al., 1994). BeWo cells also have the capability of fusing to form a syncytium and they synthesize melatonin and possess its receptors (Lanoix et al., 2006, 2008). As with numerous other cancer cells (Sainz et al., 2003; Uguz et al., 2012; Wang et al., 2012), BeWo cells respond to melatonin by undergoing apoptosis, a response that is opposite that of normal vCTB. Thus, the actions of melatonin in the placenta are clearly context specific. As already mentioned, it is common that melatonin preserves normal cells while promoting processes that cause degeneration and death of cancer cells (Choi et al., 2008; Proietti et al., 2013; Rodriguez et al., 2013).
Germane to a discussion of melatonin's action in the placenta is a report which claimed that night-time circulating melatonin levels are depressed in women suffering with severe pre-eclampsia when compared with those in women with a normal pregnancy or with mild pre-eclampsia (Nakamura et al., 2001). Since especially severe pre-eclampsia is believed to involve the excessive production of free radicals, the lower melatonin levels could be a consequence of a more rapid utilization of this free radical scavenger or a consequence of a reduced production. Also, given that the night-time level of melatonin was measured at a single time point in these three groups of women, the apparently lower values in those with severe pre-eclampsia could have been a result of a shifted nocturnal melatonin peak rather than an actual depression of its absolute concentration.
Consistent with their continuing interest in the relevance of melatonin to placental physiology, Lanoix et al. (2012b) compared the levels of melatonin, its precursor, its synthesizing enzymes and its membrane receptors in pre-eclamptic placentas with those from gestation-matched normotensive control placentas. They observed that relative to control tissues, pre-eclamptic placentas had reduced AANAT gene expression and enzyme activity along with lower ASMT activity. Correlating with these observations were dramatically depressed concentrations of melatonin and elevated levels of its precursor, serotonin. Finally, pre-eclampsia was associated with a significant reduction in both the MT1 and MT2 receptor in the placenta. In view of these outcomes, it seems clear that the markedly depressed level of melatonin in pre-eclamptic placentas is a consequence of impaired production of the indoleamine due to a major deficiency in the rate-limiting enzyme AANAT. The drop in melatonin in the pre-eclamptic placenta may help to explain the lower levels of blood melatonin in women with pre-eclampsia (Nakamura et al., 2001); however, this correlation implies that placenta-derived melatonin is normally released into the blood, perhaps especially in near-term pregnancy, when blood levels of the indoleamine reach their maximal values (Tamura et al., 2008a). Lanoix et al. (2012b) also suggested that depressed circulating melatonin levels during pregnancy may be a biomarker for the diagnosis of pre-eclampsia. They also surmise that melatonin may be useful as a treatment for pre-eclampsia.
Programming fetal circadian rhythmicity
The maternal circadian system is organized by the master clock, a small group of neurons located bilaterally at the base of the diencephalon/telencephalon junction in the anterior hypothalamus (Mason and Lincoln, 1976). These neurons, referred to as the SCN, regulate circadian rhythms of most if not all organs in the body. At the molecular level, the rhythms in the SCN are driven by a transcriptional/translational feedback loop that involves genes Per 1, Cry 1, Cry 2, Clock and Bmal1 (Bass and Takahashi, 2010); these rhythms in turn regulate circadian oscillations in peripheral organs (Menaker et al., 2013).
Clock genes have been described in two functionally distinct zones (junctional and labyrinth) of the rat placenta near the time of delivery (Wharfe et al., 2011). Clock gene expression was compared in placentas collected at four time points throughout a light:dark cycle (collected at 0800, 1400, 2000 and 0200 h). The findings revealed that although the canonical circadian genes were expressed in both placenta zones, the circadian changes were not robust nor were they well co-ordinated. Despite these data, Waddell et al. (2012) caution against discounting a potential role of circadian rhythms as a key component of the normal placental phenotype.
A primary entrainer of the cyclic function of the SCN is the light:dark cycle, as detected by the retinas. The eyes are connected to the SCN via a specialized group of axons from a select population of retinal ganglion cells (Sand et al., 2011; Lucas, 2013); the axons of these cells are referred to as the retinohypothalamic tract (Mason and Lincoln, 1976) and they course with the optic nerve to the SCN.
Axons of SCN cells project to adjacent hypothalamic neurons where they synchronize overt circadian rhythms, such as body temperature, sleeping/waking, feeding and adrenocorticotrophic hormone/corticosteroid. Additionally, via a more complex neural route involving central and peripheral sympathetic neurons, the SCN regulates the production and release of melatonin from the pineal gland (Stehle et al., 2011). Blood melatonin levels are typically 10–15 times higher at night than during the day as a result of the nocturnal production and release of the indole from the pineal gland. The maternal melatonin rhythm may have a major role in influencing the development of the fetus since unaltered melatonin readily crosses the placenta (Okatani et al., 1999; Schenker et al., 1998). Melatonin produced in organs other than the pineal gland is minimally released into the blood, but rather is used in the organ where it is synthesized as an autocoid or paracoid (Tan et al., 2003).
The circadian clocks in many peripheral organs, for example the kidney, adrenal gland, lung, are entrained by SCN-derived information supplied to them by their autonomic innervation (Bass and Takahashi, 2010). Also, daily fluctuations in body temperature and cortisol may aid in synchronizing the clocks of peripheral organs. Finally, the day/night changes in circulating melatonin cue peripheral organs of the prevailing light:dark environment (Pevet and Challet, 2011). Thus, the metabolism of cells throughout the body is exposed to a variety of circadian messages that serve to regulate gene expression. The expression of an estimated 5–15% of the genes in peripheral organs is circadian in nature (Richards and Gumz, 2012).
The maturation and synchronization of the fetal circadian system has eluded detailed examination to date. Evidence from studies conducted in humans and non-human primates has revealed entrained 24-h rhythms in fetal heart rate and respiratory movements during the latter half of pregnancy (Seron-Ferre et al., 2007). Whether the circadian system of the fetus, particularly in late pregnancy, has anything reminiscent of the pervasive influence of the maternal SCN is unknown. It has been demonstrated that the post-natal development of the peripheral circadian system in offspring of mothers lacking a functional master clock (due to lesions of the SCN or to gene knockouts) is unexpectedly relatively normal (Jud and Albrecht, 2006).
In those mammals (including the human) where studies have been done, the SCN is already morphologically distinguishable in the basal hypothalamus of the fetus by mid-gestation. Before birth it exhibits rhythms in mRNA for vasopressin (an important transmitter in the SCN) and cfos mRNA and protein (Reppert and Schwartz, 1983; Novakova et al., 2010). Moreover, the innervating fibers from the retinohypothalamic tract to the SCN are apparent before term delivery (Weinert, 2005). The oscillations that occur in the fetal SCN are typically of lower amplitude than those in the adult master clock (Watanabe et al., 2006). While the retinohypothalamic input to the SCN is apparent in the late-gestation fetus, the output of the fetal clock to the pineal gland seems to be incomplete until after birth (Reiter, 1991). Thus, there is no evidence that a fetal melatonin rhythm influences any aspect of fetal development since the pineal gland requires an innervation from the SCN via the peripheral sympathetic nervous system to produce melatonin.
Melatonin of maternal origin, however, does influence the fetus by virtue of its rhythm and the ease with which it crosses the placenta. Studies in the human have repeatedly confirmed that the cycle of melatonin in the maternal blood also occurs in the fetal circulation (Kennaway et al., 1996; Okatani et al., 1999). This rhythm, when abolished by exposure of the mother to constant light, changes the rhythmic expression in fetal clock genes; these changes are reversed when daily melatonin injections are given to the mother (Torres-Farfan et al., 2006). This documents that the fetal clock is imprinted by melatonin, which under normal circumstances is of maternal origin. The human fetus displays 24-h rhythms in temperature and oxygen consumption by 32–33 weeks of gestation (Bauer et al., 2009).
In addition to the maternal melatonin rhythm, judging from the cyclic fluctuation of cortisol levels in the umbilical artery and vein, the term fetus is also exposed to this rhythm (Seron-Ferre et al., 2001). The so-called fetal zone of the fetal adrenal gland also secretes large amounts of dehydroepiandrosterone sulfate (DHEAS), which causes a 24-h rhythm in the fetal circulation; DHEAS is a necessary precursor for placental estrogen production during pregnancy (Seron-Ferre et al., 2007). Whether there is any functional interaction of the fetal melatonin and DHEAS rhythms at the level of the fetal SCN or elsewhere is unknown.
Evidence accumulated to date suggests that the circadian architecture in the fetus, particularly in the SCN, is entrained by the maternal melatonin cycle (Fig. 5) and possibly to a lesser extent by manipulation of the feeding times (Novakova et al., 2010). As mentioned above, exposure of pregnant mothers to constant light disrupts the maternal activity cycle and suppresses the circadian melatonin rhythm; these perturbations impact gene expression in the fetal SCN. Studies in rodents have shown that maternal-generated rhythms, when disrupted during pregnancy, also alter the post-natal behavioral circadian rhythms in the offspring. For example, maternal pinealectomy, which abolishes the circadian melatonin cycle, significantly interferes with the drinking rhythm in the offspring while this cycle is restored when the pinealectomized mothers are given regular melatonin injections in late pregnancy (Bellavia et al., 2006). These observations emphasize the potential danger of constant light exposure, unusual light/dark cycles or night shift work for pregnant humans especially during the last trimester. This is certainly not trivial given that throughout the world an estimated one-fourth to one-third of female employees work at night and, moreover, considering the high degree of light pollution in urban areas, avoiding light at night sufficient to alter the function of the biological clock and circulating melatonin levels is becoming progressively more difficult.
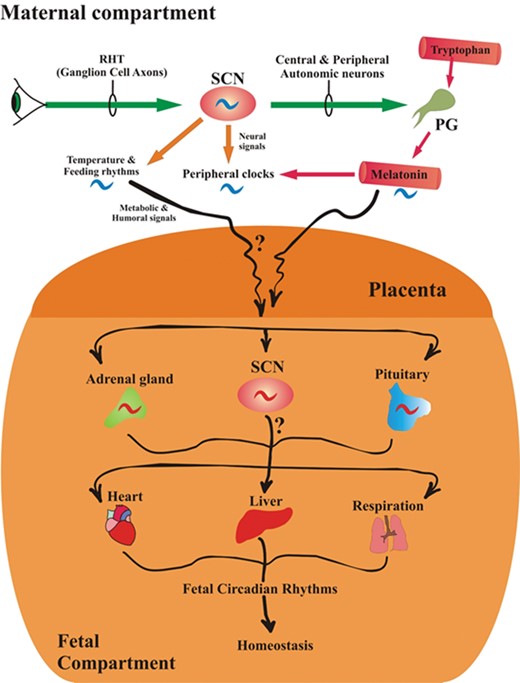
Potential routes by which the photoperiod, melatonin and the maternal master circadian clock, the suprachiasmatic nuclei (SCN), influence the development and/or maturation of the SCN in the fetal brain. The rhythmic activity of the maternal SCN is governed by the light/dark cycle. In the maternal compartment (top), the SCN controls the circadian production and secretion of melatonin from the pineal gland (PG) and imposes its activity on clocks in peripheral organs directly via neural signals and perhaps indirectly via the 24-h melatonin rhythm. The maternal SCN also influences daily changes in body temperature and feeding rhythms. Melatonin is also produced in the placenta but whether it is generated in a circadian manner in this tissue has not been determined. Also, whether placenta-derived melatonin has any bearing on fetal maturation or development is unknown. In the fetal compartment (bottom), the development and maturation of the SCN may be influenced by maternal temperature and feeding cycles and by the maternal circadian rhythm of melatonin, which easily passes through the placenta to the fetal circulation. In the fetus, these processes may also influence the adrenal corticosteroid rhythm and those of the pituitary. These organs, in turn, influence rhythms of clocks in the peripheral organs. Given that the maternal master circadian clock (SCN) is under the strong influence of the light/dark environment, it may be important that pregnant females maintain a regular light/dark cycle, especially during the last trimester. RHT, retinohypothalamic tract.
There is now general agreement that maternal circadian rhythms are influential in the entrainment and programming of fetal and newborn circadian rhythms (Fig. 5), but what rhythms are affected may be species specific. While information in this field is still rudimentary, evidence has shown that disturbances of the fetal circadian system, regardless of the cause of those perturbations, have long-term consequences in the offspring. As an example, women who engage in shift work during pregnancy have an increased incidence of spontaneous abortions, premature deliveries and low birthweight infants (Zhu et al., 2004). Shift work greatly alters the melatonin cycle, the sleep/wake rhythm and feeding times which also could be instrumental in contributing to these complications. Exposure of rats to simulated shift work caused increased hyperleptinemia and adiposity of the offspring at 3 months after birth and altered glucose tolerance and insulin resistance, reminiscent of that seen in metabolic syndrome, when the offspring were 1 year of age (Varcoe et al., 2011). Clearly, the impact of a disturbed light:dark cycle in late pregnancy and during the perinatal period may have major effects on subsequent behavioral and metabolic functions (Ferreira et al., 2012). Given the remarkable rise in the diagnosis of metabolic syndrome, obesity, attention deficit-hyperactivity disorder, autism spectrum disorders, etc., it may be worthwhile to be more attentive to the light:dark environment during pregnancy (Hardeland et al., 2012). This is especially true since the frequency of these conditions has run in parallel with excessive use of light at night, a change that is difficult to avoid in current societies.
Offspring that are delivered prematurely are not exposed to a normal maternal melatonin rhythm that they would be if they were still in utero and they do not generate a melatonin rhythm on their own (Kennaway et al., 1992). Thus, preterm infants are deprived of exposure to the melatonin cycle during a critical interval of their development. How or whether the lack of exposure to this rhythm in these premature infants has consequences on any aspect of development remains unexamined. This problem could be potentially partially rectified if premature infants would be breast fed since a melatonin rhythm normally exists in human breast milk, with higher levels at night than during the day (Illnerova et al., 1993). This still would require, however, that the mother be in darkness for several hours before and at the time of night-time breast feeding and also the much lower levels of melatonin in the breast milk (relative to those in the blood) may render these concentrations insignificant in terms of programming the SCN of the newborn.
Pre-eclampsia and placental damage: protection by melatonin
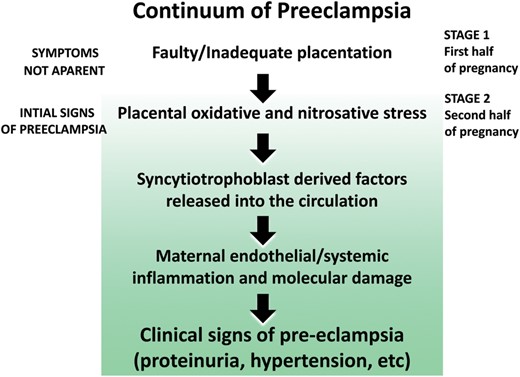
Pre-eclampsia is a dangerous complication of pregnancy which causes significant maternal and fetal morbidity and death (Hung and Burton, 2006; Nelissen et al., 2011; Redman, 2011). It is commonly diagnosed in the latter half of pregnancy but it may involve poor placentation during the earliest stage of pregnancy (Fig. 6). It can progress to an even more serious condition, eclampsia, which is accompanied by life-threatening seizures.
The potential sequence of events that contribute to pre-eclampsia. The oxidative/nitrosative stress that occurs may be a result of permanent hypoxia or intermittent hypoxia/reoxygenation resulting from placental maldevelopment because of inadequate attachment of the placenta to the uterine wall. Melatonin, due to its potent antioxidative actions, reduces ischemia/reperfusion injury to the placenta under experimental conditions and could potentially alleviate some of the signs of pre-eclampsia for the same reason. Melatonin also has antihypertensive actions which may aid in lowering the blood pressure in pre-eclamptic women. Not illustrated in this figure are the negative effects that chronodisruption has during pregnancy (see text).
Pre-eclampsia has multiple facets with a commonly observed sign being hypertension. As the condition progresses, the damaged placenta releases molecules into the circulation which cause endothelial dysfunction leading to an elevation in blood pressure (Roberts et al., 1989; Roberts and Hubel, 2009) and eventually more systemic damage, for example at the level of the kidney: these latter changes initiate proteinuria as a result of glomerular endotheliosis (Gaber et al., 1994). Several features which characterize pre-eclampsia contribute to the pathophysiology of the disease including hypertension, inflammation and oxidative/nitrosative stress. The only effective treatment for pre-eclampsia is the delivery of the fetus and the placenta, although the condition can occur during the first 2 weeks after child birth.
The role of oxidative and/or nitrosative stress in pre-eclampsia is well accepted (Myatt et al., 1996; Hung and Burton, 2006), although it is debated as to what causes the generation of the toxic oxygen derivatives that contribute to molecular damage. One theory states that free radicals and other reactive species are a consequence of a compromised uteroplacental arterial flow because of the improper remodeling of the spiral arteries during placentation (Kaufmann et al., 2003). This leads to a low oxygen tension and a relative hypoxic state in the placenta. Another view is that it is not a matter of a chronically diminished blood flow to the placenta, but rather the problem resides with the intermittency of blood flow to this tissue. This results in intervals of ample arterial blood flow followed by intervals when blood flow is inadequate, which leads to hypoxia/reoxygenation injury (Hung and Burton, 2006). These presumptive explanations differ in that in the first case the placenta is in a chronic hypoxic state while in the second situation there are repeated intervals of hypoxia followed by reoxygenation. Under both conditions, massive numbers of free radicals would be generated thereby ensuring elevated molecular damage to the uterine trophoblasts and the release of a wide range of factors that are known to be altered in pre-eclampsia that then promote inflammation. A review by Redman and Sargent (2009) describes these inflammatory agents in detail and an extensive discussion of these data is considered beyond the scope of this report.
Considering the proposed involvement of free radicals and oxidative stress as contributors to the placental damage that occurs early in pre-eclampsia, it was anticipated that antioxidants may be beneficial in ameliorating the severity or progression of this disease. When tested, however, the data do not generally support the use of either vitamins C or E as having benefit in the treatment of pre-eclampsia (Conde-Agudelo et al., 2011; Rossi and Mullin, 2011). This is also the case with some other antioxidants, for example co-enzyme Q10, that have been examined (Briceno-Perez et al., 2009).
As noted above, melatonin as an antioxidant has a much greater capacity to scavenge radicals and reduce oxidative damage than do either vitamins C or E, including in the placenta (Martin et al., 2000a; Milczarek et al., 2010). Melatonin and its secondary, tertiary and quaternary metabolites function as radical scavengers and so melatonin neutralizes many more toxic reactants than do the vitamin antioxidants (Tan et al., 1993, 2002; Galano et al., 2011, 2013). Moreover, melatonin acts as an indirect antioxidant by stimulating antioxidative enzymes (Barlow-Walden et al., 1995; Pablos et al., 1998; Rodriguez et al., 2004) and glutathione production (Urata et al, 1999), a tripeptide with significant antioxidative activity.
Since the initial assault that leads to pre-eclampsia may be oxidative damage to the placenta arising from chronic hypoxia (Kaufmann et al., 2003) or repeated hypoxia and reoxygenation (Hung and Burton, 2006) of the placental tissue, Okatani et al. (2001) examined whether melatonin would protect against placental ischemia and reperfusion. To achieve this, the authors occluded the utero-ovarian arteries bilaterally for 20 min on Day 19 of pregnancy in rats, half of which were treated with melatonin (10 mg/kg) 60 min in advance of arterial occlusion. The hypoxic/reoxygenation episode was followed by a reduction in the placental mitochondrial respiratory control index and drop in the ratio of adenosine-5-diphosphate (ADP) concentration to oxygen consumption (ADP/O) during state 3 respiration, while the level of oxidized lipids in the placenta rose. These changes were prevented when melatonin was given documenting that the indole, likely due to its antioxidant activity, is capable of protecting the placenta from ischemia/reperfusion injury. Moreover, the authors surmised that melatonin may be a beneficial treatment in other situations where free radicals contribute to fetal problems, e.g. fetal growth restriction and fetal hypoxia associated with a difficult delivery. Interestingly, subsequent reports have proved this speculation valid; melatonin reduces fetal growth restriction in rats due to either placental ischemia/reperfusion (Nagai et al., 2008) or undernourishment during pregnancy (Richter et al., 2009) and in sheep resulting from a period of ischemia followed by reperfusion (Lemley et al., 2012). In humans as well, melatonin reduced oxidative damage in newborns that experienced a period of asphyxia due to difficult delivery (Fulia et al., 2001).
Lanoix et al. (2012a, 2013) recently completed a series of in vitro studies in which they used melatonin to protect primary human STB cells from apoptosis due to 4-h hypoxia followed by 18 h of reoxygenation. The hypoxia/reoxygenation procedure clearly caused oxidative stress in the STB cells, which led to an activation of the Bax/Bcl-2 mitochondrial apoptosis pathway and DNA fragmentation. In this in vitro model, melatonin markedly reduced both apoptosis and the associated DNA damage. These findings support the possibility that melatonin may be useful in vivo to limit complications of pregnancy that involve damage to STB cells and their premature loss. For example, STB apoptosis is elevated in pre-eclampsia and intrauterine growth restriction (Heazell et al., 2008; Tomas et al., 2011).
In addition to oxidative damage to the placenta, etc., circadian misalignment may be one of many factors that contribute to pre-eclampsia (Ditisheim et al., 2013). Chronodisruption has been tentatively experimentally linked to other reproductive malfunctions including interruption of pregnancy, spontaneous abortion and low birthweight infants (Mahoney, 2010; Summa et al., 2012). The epidemiological data, however, have not uniformly supported an association between pre-eclampsia and shift work, a known circadian disrupter (Wergeland and Strand, 1997; Haelterman et al., 2007; Chang et al., 2010).
Gestational hypertension is one aspect of pre-eclampsia that may be linked to circadian disruption given that these rhythmic perturbations are also often associated with melatonin suppression. Normally, blood pressure in humans varies over a 24-h period with lowest pressure occurring at night (de la Sierra et al., 2009; Reiter et al., 2010b). This nocturnal reduction is mediated by the concurrent rise in circulating melatonin (Obayashi et al., 2013). Moreover, exogenously administered melatonin reduces hypertension in humans (Arangino et al., 1999). Thus, with the elimination of the night-time elevation of melatonin due to shift work, night-time light exposure, etc., some degree of hypertension would be expected, such as that which occurs in pre-eclampsia. Finally, placental diseases including pre-eclampsia may have an impact on the cardiovascular health of the mother later in life (Mosca et al., 2011). The cause of this association presumably relates to the endothelial damage sustained during a difficult pregnancy (Siddiqui and Hladunewich, 2011). Thus, if melatonin would have utility in reducing the severity of pre-eclampsia one downstream effect may also be an improvement of the cardiovascular health of the mother in the long term.
Pre-eclampsia is often accompanied by what is referred to as the HELLP syndrome; this condition includes hemolysis, elevated liver enzymes and low platelet count (Weinstein, 1982). Since it occurs coincident with pre-eclampsia, it is speculated to have a similar causative basis in terms of abnormal placental development or physiology (Abildgaard and Heimdal, 2013). Whether the signs of HELLP have any relation to melatonin has never been investigated. Platelets do, however, normally contain melatonin and receptors for this indole (Vacas et al., 1991; Morera and Abreu, 2005). Also, hemolysis and elevated liver enzymes are frequently a result of oxidative damage to the respective cells (Pacini et al., 2011). This being the case, melatonin, due to its antioxidant properties, may be beneficial in this condition. Finally, melatonin was observed to have anti-epileptic actions in humans (Molino-Carballo et al., 1997; Sanchez-Barcelo et al., 2011). This suggests that melatonin may reduce the likelihood of pre-eclampsia progressing to eclampsia.
Lipopolysaccharide (LPS) from the cell membranes of Gram-negative bacteria is a commonly used toxin in experimental situations to promote free radical-mediated oxidative damage. In a comprehensive molecular study designed to test whether exogenously administered melatonin would combat oxidative destruction at the level of the placenta, Wang et al. (2011) treated mice at 15 days of pregnancy with 300 µg/kg LPS without or with concurrent melatonin (5 mg/kg) treatment. Virtually every placental change induced by LPS, including depressed glutathione, elevated inducible nitric oxide synthase (iNOS), enhanced levels of 3-nitrotyrosine residues, reduced GRP78 expression, elevated elF2α and JNK phosphorylation and increased CHOP expression, was negated in mice treated with melatonin. The results of this thorough study strongly emphasize the potentially important role that endogenously produced placental melatonin could play in preserving homeostasis in this pregnancy-critical organ.
Melatonin not only protects the placenta from LPS-mediated oxidative toxicity but also, likewise, reduces the damage inflicted by LPS on the fetus. When mice were treated (on gravid Days 15–17) with LPS, the toxin increased intrauterine fetal death, caused intrauterine growth retardation (IUGR) and induced biochemical changes consistent with elevated oxidative stress in both the mothers and the fetuses (Chen et al., 2006). As in other experiments of this type, melatonin was effective in eliminating almost all the changes resulting from LPS toxicity, although it did not totally restore the reduced fetal weights of the recovered fetuses.
Other actions of melatonin at the placental level also document that the indole enhances the total antioxidative capacity of this organ. When rats that were undernourished (35% food reduction) from gestation days 15–20 were given melatonin in their drinking water, the relative expression of the antioxidative enzymes manganese superoxide dismutase and catalase were highly significantly elevated over those in the underfed animals not treated with melatonin (Richter et al., 2009). While a similar stimulation of glutathione peroxidase expression was not observed, the findings do show the likely value of melatonin in protecting the placenta from molecular mutilation by free radicals. The enzyme increases were accompanied by an improvement in the growth of the placenta (reflected in the fetal weight to placental weight ratio), which benefitted the fetuses since mean fetal bodyweights were also elevated. Thus in this case, melatonin overcame IUGR which was a result of undernourishment, a procedure believed to involve excessive free radical generation (Franco et al., 2006).
Melatonin in relation to parturition
The processes of labor and delivery of a child involve increasingly frequent and robust contractions of the myometrium accompanied by cervical effacement and expulsion of the fetus. While these events obviously occur at any time during the day or night, there are reports noting that the onset of human term labor more commonly takes place in the late night and early morning hours (Glattre and Bjerkedal, 1983; Cagnacci and Soldani, 1998; Lindow et al., 2000). Similar claims have been made relative to preterm labor (Iams et al., 2002), at least after 28 weeks of gestation (Vatish et al., 2010). During evolution there were likely selective survival advantages for both the mother and the newborn to enter parturition at night since predation pressure was lowest at that time. As in the human, similar 24-h circadian variations of birth are seen in other species (Lincoln and Porter, 1976; Zahn and Hattensperger, 1993; Farber et al., 1997).
The circadian signals that drive the nocturnal changes that culminate in the delivery of offspring in late term human pregnancy are only beginning to be unraveled (Olcese, 2012; Olcese et al., 2013). Moreover, the circadian mechanisms related to night-time parturition are probably breaking down because of the widespread misuse of light at night. Throughout evolution where the regularly recurring light:dark cycles were undisturbed, internal rhythms were more strongly coupled to the photoperiodic environment (Golombek et al., 2013). With the advent of the developments in artificial lighting, the natural environmental light/dark cycles are being markedly subverted (witness, for example, the light environments in a hospital setting) and normally entrained circadian rhythms are likewise under increasingly greater pressure. The dysynchronization of circadian rhythms, also referred to as chronodisruption (Erren and Reiter, 2009; 2013), due to perturbed or unusual light/dark cycles generally negatively influence physiological and metabolic processes and sometimes lead to disease (Blask et al., 2011; Bass, 2012; Münch and Bromundt, 2012; Golombek et al., 2013).
Within the last decade, melatonin, which exhibits a pronounced circadian rhythm in both the pineal gland and the circulation of all mammals (Vaughan et al., 1976; Panke et al., 1979; Reiter, 1986; Stehle et al., 2011), has been shown to modulate uterine physiology. With the aid of receptor autoradiography and radioreceptor assays, Schlabritz-Loutsevitch et al. (2003) identified specific high-affinity, G-protein coupled, melatonin-binding sites on the membranes of uterine myometrial cells obtained from both non-pregnant and pregnant humans. These findings are consistent with the presence of the well-defined 7-transmembrane melatonin receptors typical of many cells (Stankov and Reiter, 1990; Dubocovich and Markowska, 2005; Slominski et al., 2012). Moreover, this same group (Schlabritz-Loutsevitch et al., 2003) reported that the accumulation of cyclic adenosine monophosphate (cAMP) in cultured primary myocytes obtained from the human uterus was not suppressed by melatonin after treating the cells with forskolin. This showed that the common Gi protein coupling of the uterine melatonin receptors to adenylyl cyclase was not essential in these cells (Dubocovich and Markowska, 2005). Interestingly, however, it was found that melatonin activates the same intracellular signaling pathway as does oxytocin in uterine myometrial cells. These intracellular events include stimulation of phospholipase C, protein kinase C and myosin light chain kinase (Sharkey et al., 2009, 2010). This being the case, oxytocin and melatonin may act synergistically to promote and sustain strong uterine contractility which aids in the delivery of the fetus. This interaction between oxytocin and melatonin, which is normally elevated during darkness, could explain the higher night-time delivery of offspring (Lincoln and Porter, 1976; Zahn and Hattensperger, 1993; Farber et al., 1997). That melatonin actually sensitizes the human uterus to oxytocin is supported by the finding that cultured myometrial cells treated with a concentration of oxytocin that by itself did not induce contractions undergo powerful contractions when melatonin is added with the low oxytocin concentration (Sharkey et al., 2009, 2010).
An examination of the potential mechanisms involved in the oxytocin/melatonin synergy showed that melatonin also enhances gap junction activity and mRNA as well as protein expression for connexin 43, a gap junction protein common to many cells, including uterine myocytes. Thus, melatonin seems to assist in the co-ordination of uterine muscle cells during labor to intensify contractile strength (Olcese et al., 2013). Further examination of myometrial cells collected during labor revealed elevated 2-I125-melatonin binding compared with smooth muscle cells harvested from the non-laboring uterus. Since 2-I125-melatonin binds to both the MT1 and MT2 membrane receptors, the obvious implication is that these receptors are more abundant in the uterus during labor than during a more quiescent phase.
Preliminary studies recently performed in humans support a significant role for melatonin in aiding in the delivery of the fetus by enhancing the strength of uterine contractions (Olcese et al., 2013). Pregnant volunteers (>38 weeks gestation) were monitored continuously from 19:00–07:00 h under dim white light as to the frequency and strength of their uterine contractions. When these females were exposed to a 10 000 lux full spectrum light at 23:00 h for 1 h, the strength of their uterine contractions was diminished during the remainder of the night. This light intensity is known to suppress endogenous circulating melatonin levels (Lewy et al., 1980; Revell and Skene, 2007), although in this study melatonin was not measured. The regularity and the strength of the uterine contractions were monitored by an experienced obstetrical nurse but the study was not blinded. The results, however, generally support the idea that the nocturnal rise in melatonin contributes to the increased night-time labor onset and delivery of the offspring due to its synergistic interaction with oxytocin.
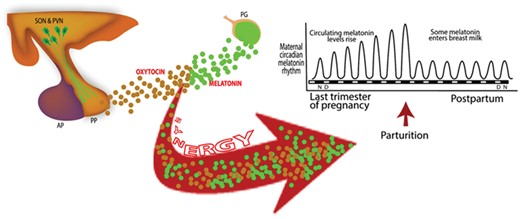
The results summarized above are intriguing in view of what is known about the melatonin rhythm during pregnancy. The amplitude of the nocturnal melatonin peak becomes steadily higher in late pregnancy such that, near term, the night-time melatonin levels are higher than those measured at any other time (Fig. 7) (Nakamura et al., 2001; Tamura et al., 2008a) thereby enhancing the likelihood of its synergy with oxytocin. This rise may be an evolutionary adaptation to enhance uterine contractions near the time when delivery should occur. Immediately after delivery, nocturnal melatonin levels in the mother return to values typical of those in non-pregnant women (Tamura et al., 2008a).
Melatonin synergizes with oxytocin from the posterior pituitary (PP) to strengthen the force of uterine myometrial contractions during labor. Since maximal melatonin levels occur at night in darkness, its synergistic actions with oxytocin may explain the elevated frequency of labor onset at night. In developed countries, the widespread use of manufactured light at night alters circadian rhythms and suppresses the nocturnal production of melatonin thereby likely reducing nighttime labor onset and spreading this process throughout the 24-h period. AP, anterior pituitary; PG, pineal gland; PVN, paraventricular nucleus; SON, supraoptic nucleus.
Several conclusions and/or recommendations follow from the data summarized: (i) considering the increasingly widespread use of manufactured light, to the point where darkness in urban areas is disappearing and humans are becoming progressively more melatonin deficient, it seems likely that chronodisruption and the loss of melatonin will remain a continuing problem; (ii) since melatonin seems to synergize with oxytocin to strengthen uterine contractions, when spontaneous labor begins melatonin could be given as a supplement to augment uterine contractions, to reduce the duration of labor and make delivery easier. Parenthetically, melatonin also may be useful during delivery since it has been shown to alleviate pain (Gitto et al., 2012; Srinivasan et al., 2012). (iii) When labor, especially during the day, is induced using oxytocin, it could be given with melatonin to intensify the strength of uterine contractions; (iv) women who have a history of early delivery (e.g. before 32 weeks of gestation) should probably avoid melatonin use during pregnancy and (v) throughout pregnancy, but especially during the last trimester, women should maintain a regular light/dark cycle, without interrupting the period of darkness with even brief periods of light, for the purpose of stabilizing their circadian rhythms and maximizing the nocturnal melatonin rise, both of which influence the maturation of the fetal circadian system.
Conclusion
Both melatonin and circadian rhythms are influential in determining optimal reproductive physiology during pregnancy. The discoveries related to melatonin production in several peripheral reproductive organs suggest that this indole has several important functions in these tissues, one of which very likely relates to its ability to obviate oxidative/nitrosative stress and placental dysfunction. The production of this highly effective antioxidant in the placenta may be of special significance since it would likely protect against conditions that involve less than optimal placentation, a situation that leads to either prolonged or intermittent hypoxia/reoxygenation and elevated free radical generation. Moreover, pre-eclampsia, which many researchers agree involves exaggerated free radical generation that leads to the manifestation of the signs of this condition, also may be ameliorated by this indoleamine. Given that melatonin readily crosses the placenta it also protects the fetus from oxidative/nitrosative stress. Considering the uncommonly low, or lack of, toxicity of melatonin, including during pregnancy (Jahnke et al., 1999), melatonin may prove to be highly valuable in optimizing the physiology of the reproductive system not only during pregnancy but also at other times (Reiter et al., 2013a). Certainly, it is the hope that research in this area will flourish during the next decade.
Circadian rhythms of the mother are seemingly highly important in programming the fetal clock, either via the melatonin signal and/or by other means. This being the case, it is imperative that during pregnancy, and perhaps especially during the last trimester, pending mothers maintain a regular sleep/wake and light:dark cycle. The light:dark cycle is especially important because the photic environment is an important impeller of the maternal master biological clock, the SCN, which in turn directly or indirectly provides circadian information to the fetus. Thus, shift work and unusual exposure to bright light at night should be avoided since they cause chronodisruption and melatonin suppression. There is a large amount of experimental and clinical data showing that disturbances caused by these processes have untoward consequences for the fetus.
Authors' roles
R.J.R. performed literature searches, had critical discussion of the data with each of the co-workers and wrote the preliminary and final drafts of the paper. D.X.T. performed literature searches, analyzed and discussed data and read drafts of the paper and made suggestions for changes/additions. A.K. and S.A.R.-C. prepared figures, discussed data and read all drafts of the paper.
Funding
No funding was obtained from any source to support this work.
Conflict of interest
The authors declare no conflict of interest.