Abstract
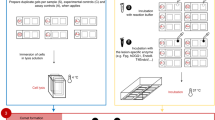
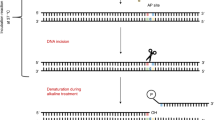
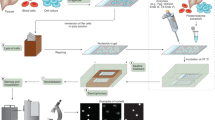
We present a procedure for the comet assay, a gel electrophoresis–based method that can be used to measure DNA damage in individual eukaryotic cells. It is versatile, relatively simple to perform and sensitive. Although most investigations make use of its ability to measure DNA single-strand breaks, modifications to the method allow detection of DNA double-strand breaks, cross-links, base damage and apoptotic nuclei. The limit of sensitivity is approximately 50 strand breaks per diploid mammalian cell. DNA damage and its repair in single-cell suspensions prepared from yeast, protozoa, plants, invertebrates and mammals can also be studied using this assay. Originally developed to measure variation in DNA damage and repair capacity within a population of mammalian cells, applications of the comet assay now range from human and sentinel animal biomonitoring (e.g., DNA damage in earthworms crawling through toxic waste sites) to measurement of DNA damage in specific genomic sequences. This protocol can be completed in fewer than 24 h.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Ostling, O. & Johanson, K.J. Microelectrophoretic study of radiation-induced DNA damages in individual mammalian cells. Biochem. Biophys. Res. Commun. 123, 291–298 (1984).
Singh, N.P., McCoy, M.T., Tice, R.R. & Schneider, E.L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 175, 184–191 (1988).
Rydberg, B. The rate of strand separation in alkali of DNA of irradiated mammalian cells. Radiat. Res. 61, 274–287 (1975).
Kohn, K.W. & Grimek-Ewig, R.A. Alkaline elution analysis, a new approach to the study of DNA single-strand interruptions in cells. Cancer Res. 33, 1849–1853 (1973).
Olive, P.L. Cell proliferation as a requirement for development of the contact effect in Chinese hamster V79 spheroids. Radiat. Res. 117, 79–92 (1989).
Olive, P.L., Banáth, J.P. & Durand, R.E. Heterogeneity in radiation-induced DNA damage and repair in tumor and normal cells measured using the “comet” assay. Radiat. Res. 122, 86–94 (1990).
Fairbairn, D.W., Olive, P.L. & O'Neill, K.L. The comet assay: a comprehensive review. Mutat. Res. 339, 37–59 (1995).
Salagovic, J., Gilles, J., Verschaeve, L. & Kalina, I. The comet assay for the detection of genotoxic damage in the earthworms: a promising tool for assessing the biological hazards of polluted sites. Folia Biol. (Praha.) 42, 17–21 (1996).
Moller, P. Genotoxicity of environmental agents assessed by the alkaline comet assay. Basic Clin. Pharmacol. Toxicol. 96 (Suppl. 1), 1–42 (2005).
Kiskinis, E., Suter, W. & Hartmann, A. High-throughput Comet assay using 96-well plates. Mutagenesis 17, 37–43 (2002).
Frieauff, W., Hartmann, A. & Suter, W. Automatic analysis of slides processed in the Comet assay. Mutagenesis 16, 133–137 (2001).
Tice, R.R. et al. Single cell gel/comet assay: guidelines for in vitro and in vivo genetic toxicology testing. Environ. Mol. Mutagen. 35, 206–221 (2000).
Bauch, T., Bocker, W., Mallek, U., Muller, W.U. & Streffer, C. Optimization and standardization of the “comet assay” for analyzing the repair of DNA damage in cells. Strahlenther. Onkol. 175, 333–340 (1999).
Banath, J.P., Kim, A. & Olive, P.L. Overnight lysis improves the efficiency of detection of DNA damage in the alkaline comet assay. Radiat. Res. 155, 564–571 (2001).
Olive, P.L., Wlodek, D. & Banáth, J.P. DNA double-strand breaks measured in individual cells subjected to gel electrophoresis. Cancer Res. 51, 4671–4676 (1991).
Collins, A.R., Duthie, S.J. & Dobson, V.L. Direct enzymic detection of endogenous oxidative base damage in human lymphocyte DNA. Carcinogen. 14, 1733–1735 (1993).
Merk, O. & Speit, G. Detection of crosslinks with the comet assay in relationship to genotoxicity and cytotoxicity. Environ. Mol. Mutagen. 33, 167–172 (1999).
Olive, P.L., Frazer, G. &p Banáth, J.P. Radiation-induced apoptosis measured in TK6 human B lymphoblast cells using the comet assay. Radiat. Res. 136, 130–136 (1993).
Ostling, O. & Johanson, K.J. Bleomycin, in contrast to gamma irradiation, induces extreme variation of DNA strand breakage from cell to cell. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 52, 683–691 (1987).
Olive, P.L., Johnston, P.J., Banáth, J.P. & Durand, R.E. The comet assay: A new method to examine heterogeneity associated with solid tumors. Nat. Med. 4, 103–105 (1998).
Olive, P.L., Durand, R.E., LeRiche, J.C., Olivotto, I.A. & Jackson, S.M. Gel electrophoresis of individual cells to quantify hypoxic fraction in human breast cancers. Cancer Res. 53, 733–736 (1993).
Olive, P.L. & Banáth, J.P. Multicell spheroid response to drugs predicted with the comet assay. Cancer Res. 57, 5528–5533 (1997).
Almeida, G.M., Duarte, T.L., Steward, W.P. & Jones, G.D. Detection of oxaliplatin-induced DNA crosslinks in vitro and in cancer patients using the alkaline comet assay. DNA Repair (Amst.) 5, 219–225 (2006).
Olive, P.L. et al. The comet assay in clinical practice. Acta Oncol. 38, 839–844 (1999).
Dorie, M.J. et al. DNA damage measured by the comet assay in head and neck cancer patients treated with tirapazamine. Neoplasia. 1, 461–467 (1999).
Speit, G. & Hartmann, A. The comet assay: a sensitive genotoxicity test for the detection of DNA damage. Methods Mol. Biol. 291, 85–95 (2005).
Strauss, G.H., Peters, W.P. & Everson, R.B. Measuring DNA damage in individual cells of heterogeneous mixtures: a novel application of an immunological typing technique. Mutat. Res. 304, 211–216 (1994).
Santos, S.J., Singh, N.P. & Natarajan, A.T. Fluorescence in situ hybridization with comets. Exp. Cell Res. 232, 407–411 (1997).
Rapp, A., Hausmann, M. & Greulich, K.O. The comet-FISH technique: a tool for detection of specific DNA damage and repair. Methods Mol. Biol. 291, 107–119 (2005).
Speit, G. & Hartmann, A. The comet assay: a sensitive genotoxicity test for the detection of DNA damage. Methods Mol. Biol. 291, 85–95 (2005).
Hartmann, A. et al. Recommendations for conducting the in vivo alkaline Comet assay. 4th International Comet Assay Workshop. Mutagenesis 18, 45–51 (2003).
Albertini, R.J. et al. IPCS guidelines for the monitoring of genotoxic effects of carcinogens in humans. International Programme on Chemical Safety. Mutat. Res. 463, 111–172 (2000).
Duez, P., Dehon, G., Kumps, A. & Dubois, J. Statistics of the Comet assay: a key to discriminate between genotoxic effects. Mutagenesis 18, 159–166 (2003).
Verde, P.E. et al. Application of public-domain statistical analysis software for evaluation and comparison of comet assay data. Mutat. Res. 604, 71–82 (2006).
Olive, P.L. & Durand, R.E. Heterogeneity in DNA damage using the comet assay. Cytometry A 66, 1–8 (2005).
Olive, P.L. & Johnston, P.J. DNA damage from oxidants: influence of lesion complexity and chromatin organization. Oncol. Res. 9, 287–294 (1997).
Sasaki, Y.F. et al. The comet assay with multiple mouse organs: comparison of comet assay results and carcinogenicity with 208 chemicals selected from the IARC monographs and U.S. NTP Carcinogenicity Database. Crit. Rev. Toxicol. 30, 629–799 (2000).
Cedervall, B. et al. Methods for the quantification of DNA double-strand breaks determined from the distribution of DNA fragment sizes measured by pulsed-field gel electrophoresis. Radiat. Res. 143, 8–16 (1995).
Olive, P.L. DNA damage and repair in individual cells: applications of the comet assay in radiobiology. Int. J. Radiat. Biol. 75, 395–405 (1999).
Speit, G. & Hartmann, A. The contribution of excision repair to the DNA effects seen in the alkaline single cell gel test (comet assay). Mutagenesis 10, 555–559 (1995).
Alapetite, C., Wachter, T., Sage, E. & Moustacchi, E. Use of the alkaline comet assay to detect DNA repair deficiencies in human fibroblasts exposed to UVC, UVB, UVA and γ rays. Int. J. Radiat. Biol. 69, 359–369 (1996).
Rydberg, B. Radiation-induced heat-labile sites that convert into DNA double-strand breaks. Radiat. Res. 153, 805–812 (2000).
Collins, A.R. The comet assay for DNA damage and repair: principles, applications, and limitations. Mol. Biotechnol. 26, 249–261 (2004).
Olive, P.L., Banáth, J.P. & Fjell, C.D. DNA strand breakage and DNA structure influence staining with propidium iodide using the alkaline comet assay. Cytometry 16, 305–312 (1994).
Acknowledgements
We thank R. Durand for developing the first comet analysis software.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Olive, P., Banáth, J. The comet assay: a method to measure DNA damage in individual cells. Nat Protoc 1, 23–29 (2006). https://doi.org/10.1038/nprot.2006.5
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2006.5
This article is cited by
-
Angle modulated two-dimensional single cell pulsed-field gel electrophoresis for detecting early symptoms of DNA fragmentation in human sperm nuclei
Scientific Reports (2024)
-
MDM2 provides TOP2 poison resistance by promoting proteolysis of TOP2βcc in a p53-independent manner
Cell Death & Disease (2024)
-
Trypanosoma cruzi infection induces DNA double-strand breaks and activates DNA damage response pathway in host epithelial cells
Scientific Reports (2024)
-
Nimbolide Exhibits Potent Anticancer Activity Through ROS-Mediated ER Stress and DNA Damage in Human Non-small Cell Lung Cancer Cells
Applied Biochemistry and Biotechnology (2024)
-
Development and validation of a human bronchial epithelial spheroid model to study respiratory toxicity in vitro
Archives of Toxicology (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.