Article Text
Abstract
Based on the idea that electric light at night might account for a portion of the high and rising risk of breast cancer worldwide, it was predicted long ago that women working a non-day shift would be at higher risk compared with day-working women. This hypothesis has been extended more recently to prostate cancer. On the basis of limited human evidence and sufficient evidence in experimental animals, in 2007 the International Agency for Research on Cancer (IARC) classified ‘shift work that involves circadian disruption’ as a probable human carcinogen, group 2A. A limitation of the epidemiological studies carried out to date is in the definition of ‘shift work.’ IARC convened a workshop in April 2009 to consider how ‘shift work’ should be assessed and what domains of occupational history need to be quantified for more valid studies of shift work and cancer in the future. The working group identified several major domains of non-day shifts and shift schedules that should be captured in future studies: (1) shift system (start time of shift, number of hours per day, rotating or permanent, speed and direction of a rotating system, regular or irregular); (2) years on a particular non-day shift schedule (and cumulative exposure to the shift system over the subject's working life); and (3) shift intensity (time off between successive work days on the shift schedule). The group also recognised that for further domains to be identified, more research needs to be conducted on the impact of various shift schedules and routines on physiological and circadian rhythms of workers in real-world environments.
- Circadian disruption
- cancer
- shift work
- epidemiology
Statistics from Altmetric.com
What this paper adds
The International Agency for Research on Cancer has classified ‘shift work that involves circadian disruption’ as a ‘probable human carcinogen, 2A.’
‘Limited’ human evidence was based on a series of epidemiological studies using crude definitions of ‘shift work’ that are difficult to compare.
This paper provides a consensus report on what aspects of shift work should be captured in future epidemiological studies.
The policy implications of an increased risk of cancer in shift workers would be complex because a large and growing proportion of the population must work a non-day shift.
Introduction
Since the advent of electric power, electric lighting has increasingly allowed for work outside the traditional dawn to dusk barrier. In fact, in modern societies, it is now a minority of the work force that are on a standard day shift schedule beginning at about 8:00 and ending at about 17:00 for 5 days a week; the majority are on non-standard work schedules including part time, weekend and work during some portion of the night.1 A fundamental aspect of mammalian biology is the circadian rhythm coordinated by the master circadian pacemaker (the endogenous clock which coordinates the molecular clocks in the rest of the organism) in the suprachiasmatic nucleus (SCN) of the hypothalamus, a brain structure responsible for linking the nervous system to the endocrine system.2 A shift in the timing of work will result in a desynchronisation of the master circadian pacemaker with peripheral oscillators, which are cell autonomous and self-sustained. This desynchronisation will persist for a variable period of time depending on shift schedule and characteristics of the individual.3 The dominant environmental factor that can reset and thereby disrupt the circadian rhythm is light at night (LAN).4 Based on the idea that LAN may increase risk of breast cancer in women and prostate cancer in men, the prediction was made that ‘shift workers’ should be at higher risk than day workers.5 Most of the epidemiological studies6–14 have reported a modestly increased risk of breast cancer in women working night or evening shifts and provided ‘limited evidence in humans for the carcinogenicity of shift-work that involves nightwork’; there is less evidence for prostate cancer. Taken together with ‘sufficient evidence in experimental animals for the carcinogenicity of light during the daily dark period (biological night),’ an International Agency for Research on Cancer (IARC) Monographs Working Group concluded that ‘shift-work that involves circadian disruption is probably carcinogenic to humans, Group 2A.’15 An important limitation of the available epidemiological studies is that there have not been clear and uniform definitions of ‘shift work’ used.
Since IARC classified shift-work into 2A, probably carcinogenic,15 there has been much scientific and public interest in the topic, and scientific interest in better epidemiological studies that could reduce the existing uncertainty in the body of studies done to date by use of improved and refined exposure assessment. This was the motivation for an IARC workshop held in Lyon on 2 and 3 April 2009. It was the purpose of the workshop to better define what is meant by ‘shift work’ in epidemiological studies of cancer, and make recommendations for improved exposure assessment. This report summarises the recommendations of that working group, supported by additional background information.
Prevalence of shiftwork and shift schedules
Early in the industrial age, three standard 8 h shift schedules were developed for many factories: day (eg, 08:00 to 16:00), swing (eg, 16:00 to midnight) or night (eg, midnight to 08:00) for 6 or 5 days followed by 1 or 2 days off. Originally this was in response to the necessity of keeping a manufacturing plant running 24 h per day. However, other reasons for shift work dominate today, such as services in a global economy, and only roughly one fourth (about 24%) of the workforce have a regular daytime, Monday-to-Friday working week1; a growing number of workers now are on 12 h shifts due in part to worker appeal because it allows for more days off per week.16 A recent survey on working conditions in Europe reported the prevalence of shift work by gender and employment type. Exposure to ‘Shift Work’ is common in the industrialised world,1 and increasing in prevalence worldwide. About 27% of the European Union work force work an evening shift five or more evenings per month, and about 10% work the night shift five or more nights per month.17 The sectors with the highest percentage of workers on a non-day shift are Hotels and Restaurants, Agriculture, Health, and Transport and Communication. Of all workers, about 6% are on a permanent non-day shift, whereas about 8% are on a rotating shift schedule. In the USA, about 15% of workers are on non-day shifts, with 3.2% on night shift and 2.5% on rotating shifts.18 Table 1 gives the relative proportions of different types of shift schedules worked by non-day workers in the European Union.
Percentages of different types of shift schedules worked by non-day workers in European Union in 2000.19 Only about one-quarter of the population is exclusively on a daytime shift
In the modern world, there are myriad shift schedules developed for a vast new array of work environments for new products and new services. There are a number of aspects of shift schedules that may be important to circadian disruption and cancer development. The first level of distinction is between a permanent shift versus a rotating shift schedule. Shifts can be rotating forward or backward, and fast or slow. Forward rotating requires day shift followed by evening followed by night, whereas backward requires day shift followed by night followed by evening. Another aspect is the number of consecutive days on the non-day shift; in general, the fewer days in succession, the less adaptation can occur, but even after a long duration of working permanent night shifts only a small percentage of workers fully adapt to a non-day circadian rhythm.
Definitions of ‘shift work’ used in previous studies
Various strategies have been implemented in the studies done to date (table 2). The two cohort analyses from the Nurses' Health Studies I and II11 12 based exposure to night work on the answer to a single question about number of years of work on a rotating shift schedule. In designing the question, the NHS researchers attempted to capture what they thought might be the most disruptive shift. So, in 1988, the question was included: ‘What is the total number of years during which you worked rotating night shifts (at least 3 nights/month in addition to days or evenings in that month)?’ As pointed out by the NHS authors, a nurse who had worked many years on a stable evening shift or stable night shift would not have included those years in answering this question. Therefore, the ‘unexposed’ group included nurses who worked many years on a non-day shift, thus possibly underestimating the impact of non-day shift work on breast cancer risk. Published in the same issue of JNCI in 2001 was a case–control study by Davis et al.6 Exposure to non-day shift work was based on a lengthy occupational history taken as part of a 70-page questionnaire administered by personal interview. The analysis included in the final publication was based only on work on the ‘graveyard shift’ (defined by the authors as beginning work after 19:00 and leaving work before 09:00) examined in three different ways: ever/never, number years with at least one graveyard shift per week, and average number of hours per week on the graveyard shift over the last 10 years. Rotation of the work schedule was asked about in one question, but the answer was recorded verbatim without any specifics as to frequency of work schedule change, or forward or backward rotation. The O'Leary et al9 study used very similar methods to that of Davis et al.6
Characteristics of studies on breast cancer regarding definitions of non-day time work
When information on the individuals' shift work history is not directly available, a possible approach, albeit an ambitious one, would be to create a job-exposure matrix for LAN exposures analogous to FINJEM20 for chemical exposures. A variation on this approach was first used on a limited scale by Hansen10 in a case–control study of night work and breast cancer from Denmark in which job title was cross-referenced with an earlier occupational survey of percentage of workers in specific job titles who worked a non-day shift in Denmark.21 For each subject (7035 cases and their individually matched controls), work history was obtained from a nationwide pension fund database, and the job titles compared with the previous survey of occupations that require work ‘predominantly at night’. Those occupations that entailed night work for at least 60% of the workers were defined as exposed, and those that entailed night work for less than 40% were defined as unexposed. However, JEM and survey-based assessments can provide only crude information about relevant exposures in shiftwork studies.
Lie et al14 used a hybrid design for exposure assessment in which cases and controls nested within a cohort of nurses were asked where they worked on a yearly basis over their nursing careers, and exposure to night work was assumed for years spent in infirmaries (except for a few departments such as managerial and outpatient), and no exposure assumed for all other nursing job locations (eg, private clinic). Though this at first appears crude, it may have been a relatively strong distinguishing feature of work for these nurses, since very few nurses (if any) in clinics work a non-day shift, whereas the great majority of nurses (perhaps all) in hospitals currently or in the past have worked at night. Pesch et al7 utilised a large case–control study of breast cancer in Germany and defined night work as a job requiring work for the entire period of 24:00 to 5:00. There was also a definition of ‘shift work’ but not ‘night work.’ Each subject was defined as exposed if she had worked one or more years at night, and ORs were based on this definition.
There was no obvious difference in results from these studies according to their varied definitions of shiftwork. They all reported significant and similarly strong associations of ‘shift work’ with risk except the studies of O'Leary et al9 and Schwartzbaum et al,13 which found no overall effect.
Definitions of ‘disruptive’ for particular non-day work history
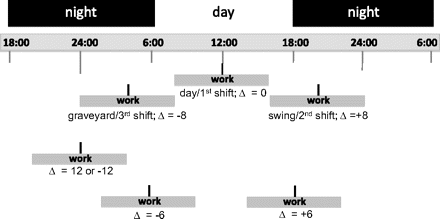
Shift work refers, in general, to a way of organising daily working hours in which different persons or teams work in succession to cover more than the usual 8 h day, up to and including the whole 24 h. In other words, a work shift can be defined in terms of the displacement of the work day from the natural solar day. The displacement statistic, Δ, is then calculated as the midpoint of the work shift minus solar noon, the midpoint of the solar day. So, for a typical day shift, Δ equals 0. For an evening, or swing, shift that begins at 16:00 and ends at midnight, Δ equals +8; and for a night, or graveyard, shift that begins at midnight and ends at 8:00, Δ equals –8. This is illustrated in figure 1. In general, if not worked one day in isolation, those shifts with a positive Δ will tend to phase-delay a worker, and those with a negative Δ will tend to phase-advance a worker.
Displacement of various shifts from solar noon; Δ=(midpoint work shift minus noon).
A phase delay occurs when an environmental influence, particularly light exposure at night, lengthens the period of the endogenous circadian rhythm by, for example, delaying the melatonin peak production; this happens, for example, when one travels rapidly west, and the sun does not set when our endogenous rhythm expects; the continued sun suppresses the beginning of the normal night-time rise in melatonin production. The ‘delay’ is physiological, and for the first few days afterwards the circadian rhythms (eg, in melatonin production, body temperature, food digestion, etc) tend to be out of synchrony with each other until these rhythms all have re-entrained to the new solar day. For travel across time zones, the new rhythm can be synchronised within a few days. However, for a shift worker, synchronisation may never occur. A phase advance occurs when the endogenous day is shortened when one travels rapidly east, and the sun sets before expected. Re-entrainment and synchronisation of the endogenous rhythms take longer for a phase advance, as in east-bound travel, than a phase delay as in west-bound travel.3 22–27 By analogy, a backward-rotating shift schedule simulates chronic phase advances, whereas a forward rotating shift schedule simulates chronic phase delays.
In fact, however, only in certain professional situations (eg, attending physicians in hospitals) will non-day shifts be worked in isolation; in most jobs they are strung together into weekly or monthly schedules, which can lead to phase-delay or phase-advance effects that persist.
If a non-day shift worker completely adapted to the new 24 h schedule, maintained this on days off and kept light exposure to only those adapted hours of wakefulness, then there would presumably be no circadian disruption and therefore no adverse health effects from it. However, due to social and societal zeitgebers (factors which can reset the endogenous circadian clock such as light during the night), this almost never happens; shift workers do not stay on a regular, though shifted, schedule of light and dark, day after day, whether working or not.
Maladaptation to a non-day shift has been discussed in the occupational literature in terms of compromised health such as heart disease,1 gastrointestinal and digestive problems, sleep irregularities including sleep deprivation, cognitive impairment and cancer.28 These result from disruption of circadian physiological organisation by working against our endogenous circadian rhythms. A particular shift can be defined according to the solar day, as indicated above, and also according to level of circadian disruption. Circadian disruption is characterised by at least two inter-related issues, melatonin suppression (which may or may not induce phase shifting), and phase shifting and the attendant desynchrony of the master pacemaker with the sleep cycle and with the peripheral oscillators in tissues throughout the body.
The first, melatonin suppression, may have many direct and indirect physiological effects that could raise cancer risk29 including alterations in hormone levels, such as oestrogens, that are known to affect risk of cancer. The second may be linked to clock gene influence on expression of genes in tissues for cellular processes (cell-cycle regulation, DNA repair, apoptosis, etc) that influence the chance that a normal cell will become transformed into a cancer cell. The two aspects might work together in which clock gene alteration results in a normal cell transforming into a cancer cell, and then melatonin suppression resulting in release of cancer cells from growth inhibition through oestrogen signalling,30 or increased linoleic acid availability to cancer cells in a small tumour that would otherwise have remained indolent.31 Another related possibility is that the sleep disruption and deprivation in non-day workers contribute to cancer risk. This might occur from a couple of mechanisms including effects on immune function32 or metabolism.33 34
A single acute light exposure during the natural dark period causing melatonin suppression may not result in a phase shift of the circadian rhythm. It will require repeated night-time light exposures, as in a non-day shift work occupation, to result in a phase shift and desynchronisation. However, each acute melatonin suppression may result in a transient alteration in SCN signalling and a potential transient decoupling of the clock-controlled genes from their normal function. Accumulated over many years, these chronic decouplings might increase disease risk.
An emerging area of interest is in the potential role of circadian gene variants in cancer risk.35 These variants may also influence susceptibility to shift work maladaptation. Another emerging area is in epigenetic reprogramming of circadian genes such as by promoter methylation or chromatin remodelling.36 37
Additional circadian considerations
Life under a solar illumination schedule (ie, without electricity) follows a temporal organisation of the many circadian clocks in cells and tissues. Whereas the SCN provides a link of retinal light exposure to tissues of the body,38 39 and functions thereby as a ‘master circadian pacemaker,’ circadian genes are present in all cells of the body, and different tissues coordinate their activity in a circadian fashion that also takes account of other factors depending on the tissue function such as timing of meals for the gut.40 The circadian oscillations in physiology in tissues are kept in step by humoural and/or neural signals from the SCN and the pineal hormone melatonin, the secretion of which is also light–dark-dependent.41 However, under conditions of circadian disruption as by non-day work schedules, these many tissue rhythms become out of synchrony and re-entrained at different rates.42–46 This adds complexity assessing the degree of circadian disruption in shift work in epidemiological studies due to other factors affecting these rhythms.
Finally, there may be interactions of circadian rhythms and other endogenous rhythms of longer duration on the degree of biological disruption and effects on cancer risk.47–52
Sallinen and Kecklund16 review the evidence on impact of various aspects of shift work that influence sleep quality and sleepiness on the job. Although it is unclear how closely sleep quality is related to circadian disruption that could increase cancer risk, these studies do offer some insight into the biological disruptiveness of night work. In addition to the clear difference between forward- and backward-rotating shifts, they reported that shifts requiring very early morning start times were deleterious to sleep, as were shift schedules which required many days in succession of night work, with short periods in between. A switch to a 12 h shift schedule was not substantively more sleep-disruptive than 8 h shifts if there were at least several days off between the 4-day work periods. Workers on regular shift schedules, even when rotating, suffered less sleep disturbance than workers on irregular schedules. Interestingly, they reported that permanent night workers had significantly poorer sleep than day or evening workers, and only marginally better than rotating shift workers. Although there is undoubtedly a self-selection of permanent night workers on social and perhaps on genetic grounds (night workers report evening preference), only a small group of such workers (<5%) show a complete adaptation to night work.
Shift domains to be captured in epidemiological studies
There are a number of domains of a shift and shift schedule that the working group believes to be important to capture in future epidemiological studies of cancer (summarised in table 3). These were developed during the course of the discussions at the workshop, and in the development of this paper. The major domains are:
Domains for capture in epidemiological studies.
shift system (start time of shift, number of hours per day, rotating or permanent, speed and direction of a rotating system, regular or irregular);
years on a particular non-day shift schedule and cumulative exposure to the shift system over the subject's working life;
shift intensity (time off between successive work days on the shift schedule).
These domains are based in part on the biological considerations outlined in previous sections such as the fact that adaptation can occur more quickly after a phase delay than a phase advance. This would suggest that a forward-rotating shift is less disruptive than a backward rotation, though both are presumably more disruptive than a stable shift.
The column called ‘Variable’ is meant to convey the features of the Work Domain that the working group believed to be important to assess for meaningful epidemiological studies of cancer to be conducted. The less of these variables that are captured, and the less accurate the information on each, the less valid will be the study; exposure misclassification will increase rapidly as the detail on the Work Domain decreases.
A diagnosis of cancer is the culmination of many years, or decades, of accumulated damage to cells and tissues; although recent exposures can contribute to the growth of a tumour, the occurrence is often dependent on the many years beforehand in which a level of damage has already accumulated. The variables of start and stop time of shift, rotating or not, and number per month are meant to reflect the intensity, or rate at which potential damage occurs, whereas duration reflects the lifelong burden of the non-day shift. It is not yet clear precisely what combination of intensity and duration is the most harmful in causing cancer.
Much more needs to be learnt about how various shifts and shift schedules affect circadian rhythmicity in real workers in real-world work environments; this is discussed in the next section below. The suggestions in table 3 are meant to provide some assessment now of the degree of circadian disruption experienced by a worker from their occupational shift history, and also to provide data for further refinements of this assessment based on new research in the field on circadian effects of various job shift requirements. If extensive information is collected now in current studies, it may be used in later, and possibly pooled, analyses that better define the disruptive characteristics of work. Good-quality exposure data on shiftwork, LAN, circadian disruption and other relevant factors can also be collected retrospectively in nested case–control studies.
Effect modification
The putative effect of shift work on cancer may be modified by an individual's ability to adapt to different shift schedules (eg, morning/evening type) and clock gene polymorphisms. Further, there are known genetic polymorphisms in detoxifying enzymes that change an individual's sensitivity to exposure to a toxic chemical.53 Similarly, there may be significant differences in susceptibility to adverse effects from chemical exposures in non-day workers compared with day workers. This is based on the known circadian variations in DNA excision repair,54 and in cell proliferation and activity of detoxifying enzymatic capacity.55–57 These variations by time of day have begun to be exploited in delivery of cancer therapy (chemicals or radiation) to optimise killing of cancer cells while minimising damage to normal cells,56 57 but the important possibility that time of day of occupational exposures could affect risk has not been investigated to date. These presumably would depend upon the biological time (ie, circadian rhythm stage) which in shift workers may not coincide with the clock hour experienced in day workers.
Related to this is the effect modification which might exist whereby ‘evening type’ persons who better tolerate night work than ‘morning type’ persons according to their delayed circadian phase position may have less disruption of their biological rhythms and therefore a smaller increase in risk of cancer. Chronotype can be measured and analysed in many study designs58 and should be included when possible in studies of shift work.
New research directions on shift disruption
There is a vast occupational literature on the health effects, and relative adaptive success for shift workers engaged in a wide array of shift schedules focused on social and physical problems, safety on the job and cognitive performance. This literature has for the most part not focused on circadian disruption per se as it might relate to cancer risk. Of the biomarkers studied so far—body temperature, cortisol and melatonin—the latter seems to be the most promising in terms of sensitivity and specificity with regard to circadian disruption59 60; cortisol may also be of value,61 although it is known to be affected by other conditions such as stress. While plasma and salivary melatonin are excellent biomarkers of current melatonin levels, the urinary metabolite 6-sulfatoxymelatonin has the advantage to better reflect an individual's melatonin level over the period since last urination; for the morning void, this would include most of the nocturnal hours. Several studies on shift work and melatonin levels have been conducted,62–65 but the best timing for sampling of urinary melatonin, that is during a period of normal working hours, before a night shift, after the most disruptive shift or after the most representative shift, is currently not well understood.
Therefore, it is recommended that workers engaged in specific shift work schedules be recruited into cross-sectional or short-term longitudinal studies according to the degree of their circadian disruption. This would include extensive melatonin measurements in urine and saliva during work days and days off, both for assessment of total melatonin production and for assessment of degree of desynchrony of circadian phase with sleep and social activity. Questionnaire data on detailed working hours, lighting intensities at night and leisure time activities could be supplemented by actimetry data gathered from small wrist-worn measurement devices. A single determination of a biomarker may have limited validity due to intraindividual variation, so that samples taken at multiple time points would be preferable. A statistic developed by Burch et al,62 the sleep-to-work urinary melatonin ratio, may play a valuable role in this work since it is simple yet possibly highly informative. Rea et al66 utilised the ‘Daysimeter’ to assess the alignment of circadian light exposure and activity in day-working nurses compared with rotating shift nurses, and found pronounced differences. In addition, assessment of circadian gene expression is possible and might provide novel insight into circadian regulation (eg, by assay of these genes in circulating lymphocytes), and may differ according to shift schedule and vary by time of day.
From this work will come a better understanding of the relative impacts of different shifts and shift schedules on circadian physiology that could be used to rank study subjects on ‘exposure’ and further improve the exposure assessment. The choice of biomarker must depend on study design. For case–control studies, any measured level of a hormone or metabolite may be seriously compromised by the disease status and/or the therapy. This is particularly true in studies on shift work, as subjects will have changed their working behaviour due to their disease; the markers are all short-lived and only reflect recent exposure. However, for studies of genetic polymorphisms in circadian genes, the case–control approach is powerful. For prospective cohort studies in which samples can be gathered before disease occurrence, there are many candidate biomarkers starting with melatonin and other hormones. In addition, markers of immune function may be informative.
There is evidence that breast tumour cells have altered circadian gene expression when compared with surrounding normal cells,67 although this may be a result of the disease process and not its cause. Epigenetic changes, for example promoter hypomethylation of CLOCK, in peripheral lymphocytes have also been reported to be associated with breast cancer risk.68 It has been shown that environmental exposures can cause DNA methylation changes,69 and these can be reversible.70 Hence, another important question is which environmental factors can result in altered promoter methylation in CLOCK and other circadian genes.
Conclusion
Cancers of breast and prostate are the two most common cancers in women and men respectively. There is mounting evidence from human and animal studies that shiftwork involving circadian disruption may be an important risk factor.15 71 Future studies should ensure that the measurement of shiftwork incorporate as many relevant factors as possible and that the metrics used be comparable across studies. The working group could not recommend any one study that would settle the issue of cancer risk of non-day work. The working group did favour the development of prospective studies in which as many of the domains shown in table 3 are captured as possible from employee questionnaire and from company employment records. Although optimum, prospective studies are not always feasible, and carefully conducted case–control, and other, designs could also yield fruitful information.
As the IARC and the working group recognise, no one study can, by itself, ‘prove’ cause and effect, nor can a group of studies of the same epidemiological design persuasively rule out bias and/or confounding. According to the IARC Preamble, ‘sufficient evidence of carcinogenicity’ from the human studies requires that a positive association has been established and that chance, bias and confounding can be ruled out with reasonable confidence. For this to occur, a varied epidemiological approach utilising case–control, cohort, and ecological designs is needed. There are also a wide, and growing, array of settings in which non-day work is now common, and in which studies of shift work and cancer should be conducted.
Unlike other common cancers, major occupational or environmental causes of breast and prostate cancers have not been identified. If the increasing use of electricity to light the night is a major determinant, then studies of shift workers provide perhaps the most powerful epidemiological tool to quantify this risk.
References
Footnotes
Funding The workshop was funded by the Health and Safety Executive (HSE), UK, and the German Social Accident Insurance (DGUV). The two sponsors were represented at the workshop by A Cassidy (HSE), F Jahn and FP Bochmann (DGUV).
Competing interests None.
Provenance and peer review Not commissioned; externally peer reviewed.