Article Text
Abstract
OBJECTIVE To examine the risk of parkinsonism related to lifetime occupational exposure to pesticides among a cohort of men, mostly orchardists, in Washington State.
METHODS All 310 subjects in this study had previously participated in a cohort study of men occupationally exposed to pesticides. Subjects were given a structured neurological examination and completed a self administered questionnaire which elicited detailed information on pesticide (insecticide, herbicide, and fungicide) use throughout their working careers. Demographic characteristics were also sought. Subjects had a mean age of 69.6 years (range 49–96, SD 8.1). There were 238 (76.8%) subjects who reported some occupational exposure to pesticides, whereas 72 (23.2%) reported none. Parkinsonism was defined by the presence of two or more of rest tremor, rigidity, bradykinesia, and impairment of postural reflexes in subjects not on antiparkinsonian medication, or the presence of at least one sign if they were on such medication. Parkinson's disease was not studied explicitly because of the difficulty in distinguishing it from other parkinsonian syndromes. A generalised linear model was used to estimate prevalence ratios (PRs) for parkinsonism relative to history of farming, pesticide use, and use of well water.
RESULTS A PR of 2.0 (95% confidence interval (95% CI) 1.0 to 4.2) was found for subjects in the highest tertile of years of exposure to pesticides; a similarly increased, non-significant, PR was found for the middle tertile (1.9 (95% CI 0.9 to 4.0)), although a trend test did not show a significant exposure-response relation. No increased risks were found associated with specific pesticides or pesticide classes, nor with a history of farming or use of well water.
CONCLUSION Parkinsonism may be associated with long term occupational exposure to pesticides, although no associations with specific pesticides could be detected. This finding is consistent with most of the publications on this topic.
- farmer
- parkinsonism
- pesticides
Statistics from Altmetric.com
Parkinsonism is characterised by rigidity, rest tremor, bradykinesia, and impairment of postural reflexes, and is primarily due to decreased function of the extrapyramidal system.1 2 Parkinsonism has been associated with several chemical exposures, including manganese and other metals, carbon disulfide and other organic solvents, and carbon monoxide.1 3
Despite much scientific interest and the passage of nearly 2 centuries since its first recognition, little is known about the cause of idiopathic Parkinson's disease (PD), which is a distinct disease entity among parkinsonian syndromes. Nevertheless, mounting evidence suggests an aetiological role of environmental factors. The first such evidence was the discovery of severe parkinsonism remarkably similar to PD in intravenous drug misusers exposed to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP).4 This condition was caused by degeneration of dopaminergic neurons in the nigrostriatal pathway similar to that found in idiopathic PD, a fact which distinguished it from other agents that can induce parkinsonism. Structural similarity between MPTP and the herbicide paraquat5 has led to speculation of a possible association between exposure to pesticides and PD. Also, several studies have suggested that rural living, use of well water, farming, and pesticides are risk factors for PD6-25 or parkinsonism.26 Limited evidence for heritability in typical PD in a recent large study of twins further supports the notion of an environmental aetiology.27
The present study examined parkinsonism relative to lifetime occupational exposure to pesticides among participants in a previous cohort study of men exposed to pesticides in Washington State. Parkinsonism was defined as the presence of two or more of the following signs: bradykinesia, rest tremor, rigidity, and impairment of postural reflexes. This study did not attempt to distinguish idiopathic PD from other forms of parkinsonism, which can be caused by various non-agricultural chemicals and medications, or may occur as a secondary effect in Alzheimer's disease or after stroke.
Methods
SUBJECTS
All subjects had previously participated in a cohort study carried out by the Washington State Department of Health during 1972–6.28 Subjects exposed to pesticides in that study consisted of orchardists (n=739, 56.8%), professional pesticide applicators (n=30, 2.3%), pesticide formulation plant workers (n=7, 0.5%), and other farm or agricultural workers (n=6, 0.5%). The “Polks Wenatchee City Directory” was used to identify non-exposed subjects (n=504, 38.8%) whose occupations entailed similar physical activity as the exposed subjects. Non-exposed subjects were frequency matched with exposed subjects by age, race, and degree of occupational physical activity, and consisted primarily of construction workers, lorry drivers, road workers (not applying herbicides), roofers, painters, plumbers, non-agricultural labourers, welders, telephone workers, salesmen, custodians, mechanics, linemen, fire fighters, veterinarians, and foresters. Occupation at the time of enrollment into the original cohort could not be ascertained for 14 (1.1%) subjects. Subjects were men, mostly non-Hispanic white (95.8%), and aged 18–88 at the time they entered the original study in the 1970s.
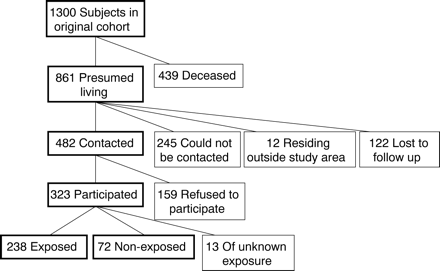
Of the original 1300 cohort members, 439 (33.8%) were dead, leaving 861 (66.2%) presumed to be living (fig 1). With information from various sources, including contact information provided by subjects in the original study, we located and attempted to contact 739 subjects (56.9% of the total cohort, 85.8% of the subjects presumed to be living). Twelve subjects (0.9% of the cohort total) were found to be living outside of Washington State and were, therefore, not included in the present study, leaving 727 potentially eligible subjects (55.9% of the total cohort). We successfully contacted 482 (66.3% of these potentially eligible subjects) and invited them in the summer of 1997 to participate in the current study; 323 (67.0% of subjects successfully contacted) accepted. The 245 subjects presumed to be living who could not be contacted for follow up were similar in age (mean age 70.6 years) to participants in the present study (mean age 69.6 years), but were slightly less likely than participants to have reported occupational use of pesticides in the original cohort study (76.6% v 83.5%, respectively). The 159 subjects who refused to participate in the present study were also similar in age (71.2 years) to participants, but were also less likely to have reported occupational use of pesticides in the original study (69.8%). Study participants underwent a 3–4 hour assessment at a centrally located testing centre. The study protocol was approved by the University of Washington and Oregon Health Sciences University Human Subjects Committees, and all participants provided written, informed consent.
Subject selection in the present study.
EXAMINATION
A nurse trained in neurological examinations administered a 20 minute structured, uniform neurological examination to all subjects. The presence and severity of motor signs were recorded by the nurse with the unified Parkinson's disease rating scale (UPDRS),29 which can be reliably administered by trained nurses.30 Signs included postural, action, and rest tremors, rigidity in each limb, impairment of postural reflexes, bradykinesia, as well as speech impairment, hypomimia, and slowness of finger taps (repeated tapping of thumb with index finger), hand movements (repeated opening and closing of hands), rapid alternating movements (repeated pronation and supination of hands), rising from a chair, and gait. The nurse was blinded to the exposure of the subjects.
ASCERTAINMENT OF USE OF PESTICIDES
Each subject was asked to complete a self administered questionnaire which included detailed questions about the subject's use of pesticides (insecticides, herbicides, and fungicides) throughout his working career. Subjects were asked to provide information on years of farming or employment involving pesticides, and for discrete periods: crops grown; number of acres of each crop; use (mixing, loading, or applying) of specific insecticides, herbicides, and fungicides (provided in a comprehensive list appropriate to the region); target crops for each pesticide used; typical methods of application; and use of protective clothes and equipment. Most of this information was collected by calendar decade; use of specific pesticides was collected by 5 year period as far back as 1960, and open ended before then. Subjects were also asked to report any pesticides not included in the list provided. Demographic information, including age, race, health conditions, use of medications, history of alcohol consumption, and history of smoking were collected. An interviewer administered the questionnaire to the few subjects (n=6) who were unable to read it for any reason. Thirteen subjects provided insufficient information with which to assess their histories of exposure to pesticides; thus, a total of 310 subjects were included in the following analyses.
DATA ANALYSIS
Exposure to pesticides was assessed in several ways. These included the dichotomous measures of (a) any history of farm employment; (b) any history of use of insecticides, herbicides, and fungicides, as well as the more specific categories of carbamates, organophosphates, organochlorines, dithiocarbamates, and pesticides containing manganese (which are also included in the dithiocarbamate category); and (c) any history of use of specific pesticides—such as azinphos methyl, DDT, diazinon, ferbam, lead arsenate, mancozeb, maneb, methyl parathion, paraquat, tetraethyl pyrophosphate (TEPP), thiram, zineb, and ziram (most of which are contained in the chemical classes already mentioned). The categories in item b were created by grouping the appropriate individual reports of pesticides. We also considered continuous measures, grouped into tertiles, of (d) years of exposure to, or use of, the factors already mentioned; and (e) number of acre-years of use of the previously mentioned pesticides (number of years of use×number of acres of the particular crop(s) to which it was applied). Analyses involving continuous measures of exposure were restricted to subjects reporting some use of the appropriate chemical or chemical class to reduce heterogeneity in factors not related to exposure (subjects with the lowest non-zero exposure were used as the reference group).31 As before 1960 information on use of pesticides was only “yes” or “no” for each pesticide, the first year of use for a pesticide before 1960 was assumed to be either the year of introduction of the pesticide or the first year of farming of the subject, whichever came later. Specific pesticides and pesticide classes were chosen for analyses based on their potential neurotoxicity—for example, pesticides containing manganese, dithiocarbamates which can produce carbon disulfide, and paraquat because of its structural similarity to MPTP. Because there was appreciable overlap in the patterns of exposure to pesticides reported by the different occupationally exposed groups and because excluding non-orchardists from the analyses had little effect on results, analyses included all exposed subjects.
Parkinsonism was defined based on the presence of the four cardinal signs: rest tremor, rigidity (in any limb), bradykinesia, and impairment of postural reflexes. A sign was considered present if it occurred at a level of slight or greater (rating ⩾1) on the UPDRS, except where otherwise noted. Subjects were considered to have parkinsonism if they had two or more of these signs, or if they had one sign and were on antiparkinsonian medication. These criteria have been used in two other studies of parkinsonism,32 33 whereas another study used overlapping, but more conservative, criteria.34
Prevalence ratio analyses were performed on all exposure measures for which there were sufficient cases of parkinsonism. Because of the high prevalence of parkinsonism among these subjects, we estimated prevalence ratios (PRs) and 95% confidence intervals (95% CIs) using a generalised linear model with binomial distribution and a log link function.35 Adjustment was made for age (<65, 65–74, ⩾75 years) and pack-years of cigarette smoking (continuous linear). An increased or decreased PR was considered to be significant when the 95% CI did not include the null value. All analyses were performed with SAS statistical software.36
Results
The 310 subjects in this study had a mean age of 69.6 years (range: 49–96, table 1). Non-exposed subjects were slightly younger than exposed subjects, with mean ages of 67.4 and 70.2 years, respectively. As would be expected, exposed subjects with the fewest years of exposure were younger on average (68.8 years) than those with the most years of exposure (72.5 years). All subjects were male; 297 (95.8%) were non-Hispanic white. A large proportion (42.6%) reported consuming less than one alcoholic drink a month, and a similar proportion (47.1%) reported being current alcohol drinkers. These proportions were similar across exposure subgroups. Although one third of the subjects had never smoked cigarettes, almost half reported 10 or more pack-years of smoking, and 23.9% reported over 35 pack-years. Non-exposed subjects tended to have more pack-years of smoking than exposed subjects. Only one subject (0.3%), an orchardist, reported being diagnosed with PD by a physician.
Selected demographics of subjects (n (%))
We found rest tremor of slight or greater severity in seven (2.3%) subjects (table 2). Although the number of cases with rest tremor was very small and the prevalence estimates consequently unstable, this measure seemed to be more common among subjects with a longer duration of exposure to pesticides. Rigidity was very common in this sample, occurring in 217 (70.0%) subjects, and being slightly more common in the arms than in the legs. Prevalence of any rigidity was similar between exposed and the non-exposed groups, but was higher among the most exposed (79.3%) compared with the least exposed (63.9%) group. For individual limbs, rigidity was consistently higher in the most exposed compared with the less exposed groups. Bradykinesia, found in 54 (17.4%) subjects, was found more often among the non-exposed than the exposed groups, but showed a significant positive trend, after adjustment for smoking and age, with years of exposure among the exposed groups. Impairment of postural reflexes was present in 39 (12.6%) subjects and showed no association with exposure. These signs contributed to an assessment of parkinsonism, as defined in this study, in 65 (21.0%) subjects. Prevalence of parkinsonism increased with increasing years of exposure. The prevalence was higher in the non-exposed group than in the least exposed group, but was similar between the non-exposed and the most exposed groups. Although reports of effects of acute exposures (poisonings) increased with increasing years of exposure, episodes of pesticide poisoning were unrelated to parkinsonism.
Prevalence (n (%)) of individual parkinsonian signs (of slight or greater severity, UPDRS rating ⩾1)
No significant associations were found between parkinsonism and ever use of well water, farm employment, or ever exposure to any specific pesticides or pesticide classes when adjusted for age and smoking (table 3). The number of cases with exposure to pesticides containing manganese (mancozeb and maneb, ferbam, thiram, and zineb) was very small, preventing further analyses of these agents.
Parkinsonism prevalence ratios (PRs, ever or never) by exposure related to pesticides
Analyses by tertiles of years of exposure showed a marginally significantly increased adjusted PR (95% CI) for the highest tertile of general exposure to pesticides (2.0 (1.0 to 4.2), table 4). The PR for the middle tertile of this exposure was increased but not significant, and a trend test was also not significant (p=0.17). The middle tertile of exposure to azinphos methyl had a significantly reduced adjusted PR (0.5 (95% CI 0.2 to 1.0)), although no significant effect was found in the upper tertile. Significant duration-response relations, as assessed by tests of trend, were found only for crude analyses of exposure to pesticides, insecticides, and lead arsenate. We found no significant associations between parkinsonism and acre-years of any category of pesticide (table5).
Parkinsonism prevalence ratios (PRs) by tertiles of years of exposure related to pesticides
Parkinsonism prevalence ratios (PRs) by tertiles of acre-years of exposure related to pesticides
Because owners of small farms may be more likely to apply pesticides themselves, and thus potentially receive greater exposures than owners of large farms, we repeated the analyses restricted to farmers whose acreage averaged over time was in the bottom tertile and also those farmers whose acreage averaged over time was in the upper tertile (data not shown). Results were generally similar to those found for the full cohort. However, we did find a significantly increased adjusted PR (95% CI) for the middle tertile of years of exposure to organochlorines (9.5 (1.3 to 69.4)) among farmers with average acreage in the upper tertile. The highest tertile of exposure to organochlorines also showed a substantially increased, but non-significant, PR (95% CI) (4.1 (0.5 to 33.9)).
An unusually high prevalence of rigidity was found in these subjects, suggesting possible overascertainment by the nurse administering the neurological examination. Therefore, the analyses were repeated with a minimum cut off of mild (UPDRS rating ⩾2) for severity of rigidity (requiring that rigidity be mild or greater when assessing slight or greater parkinsonism). This reduced the adjusted parkinsonism PRs (95% CIs) for ever versus never exposure to general pesticides (0.5 (0.1 to 3.2)), dithiocarbamates (0.5 (0.3 to 1.1)), and pesticides containing manganese (0.2 (0.0 to 1.3)), although these estimates remained non-significant (data not shown). The highest tertile of years of exposure to herbicides was the only measure of duration to show a significant association with parkinsonism, with an increased adjusted PR (95% CI) of 2.5 (1.0 to 6.0). For years of exposure to pesticides, the adjusted PR (95% CI) for the middle tertile decreased to 1.2 (0.4 to 3.6), but remained increased, albeit non-significantly, for the highest tertile at 2.0 (0.8 to 5.2). No appreciable changes were found for acre-years of exposure. We also repeated the analyses considering rigidity to be present only if found in at least two limbs (data not shown). The adjusted PR (95% CI) for the highest tertile of years of exposure to pesticides decreased slightly and became non-significant (1.7 (0.8 to 3.6)). Other PRs changed very little.
Results were similar when analyses were also adjusted for history of alcohol consumption (current, former, never) or when we excluded heavy alcohol consumers (defined as either (a) ⩾7 drinks a drinking session or (b) ⩾3 drinks a session and ⩾1 session a day, data not shown). Exclusion of subjects with other factors which might affect performance on some components of the UPDRS produced results that were mixed, but generally consistent with what was found for the full cohort. Excluding subjects with any history of stroke resulted in significantly increased adjusted PRs (95% CI) for medium and long term exposure to pesticides (2.8 (1.1 to 7.1) and 3.0 (1.2 to 7.5), respectively) and for long term exposure to insecticides (2.3 (1.1 to 4.9)). Excluding people reporting neck or back disorders also increased the adjusted PRs (95% CI) for medium and long term exposure to pesticides to 2.4 (0.9 to 6.3) and a significant 2.6 (1.0 to 6.5), respectively. Exclusion of subjects with nerve problems or neurological disorders of known aetiology (diabetes, cerebral trauma, carbon monoxide poisoning, carpal tunnel syndrome) resulted in non-significantly increased adjusted PRs (95% CI ) for both medium and long term exposure to pesticides (2.3 (0.9 to 5.8) and 2.1 (0.9 to 5.4), respectively). Excluding subjects with arthritis diagnosed by a physician had no appreciable effect on the results.
Discussion
This study suggests that parkinsonism, as defined in this study, may be associated with long term occupational exposure to pesticides. However, we found no increased risk of parkinsonism associated with specific pesticides, nor with general farm employment or with use of well water.
Our focus on pesticides relative to parkinsonism was motivated in part by the frequent finding of higher rates of PD associated with rural living or use of well water.6 7 10 14 19 20 24 25Other motivating factors were the recognised neurotoxicity of some widely used organophosphate pesticides,37 the similarity of chemical structure between MPTP and the herbicide paraquat,5 and reports of parkinsonism induced by exposure to certain pesticides.38 39
A study of parkinsonism, as opposed to the more specific PD, among kibbutz residents in Israel26 found increased risks of extrapyramidal signs among subjects with a history of field crop and landscape work. The association was particularly strong for a history of field work with cotton, which typically requires more pesticides than most other crops. Related research by these authors suggests that these extrapyramidal signs may represent an early stage of PD.40 41
Research by other investigators suggests a link between exposure to pesticides and idiopathic PD. Similar to our results involving parkinsonism, Hertzman et al 13found a doubling of risk of PD among men with occupational exposure to pesticides—primarily as orchardists—but were unable to associate the increased risk with any specific pesticides. An earlier study by Hertzman et al 12 found increased risks of PD from working in orchards. Several authors have reported an increased risk associated with farming and exposure to insecticides and herbicides, but not fungicides.7 11 14One study found a positive exposure-response gradient between duration of use of herbicides or pesticides and risk of PD.18Semchuk et al 22 found an increased risk only for herbicide use. A recent large case-control study from Germany21 is one of the first to identify specific chemical classes of pesticides, including organochlorines and alkylated phosphates, as risk factors for PD.
Despite the chemical similarity of paraquat and MPTP, studies of the association between paraquat and PD have produced mixed results. Hertzman et al 12 found a significant positive association among a few subjects exposed to paraquat, although a later study by these authors13 and another study of early onset PD20 found no association. The strongest evidence comes from a study by Liouet al 18 that reported an exposure-response relation between years of use of paraquat and risk of PD. These inconsistent results may be due to differences in study populations or to recall bias in these case-control studies.
Our study has several potential biases and limitations. Subjects with parkinsonism may be more likely than non-cases to remember or overreport use of pesticides, although this is unlikely for two reasons. The first is that an increased risk of parkinsonism was found only for general use of pesticides and not for any specific pesticides. Furthermore, most of our subjects with parkinsonism showed only slight signs of parkinsonism and did not seem to be substantially impaired by it. Any recall error was likely to be non-differential, given the many pesticides reported, the complex temporal pattern of their use, and the fact that subjects were not informed of the study hypotheses.
There is a possible volunteer bias in this study, even though these subjects belong to a cohort that was established in the early 1970s, before most of them were likely to show signs of parkinsonism. Both unlocated subjects and those who were contacted but refused to participate were similar in age to participants in the present study, but were slightly less likely to have reported occupational use of pesticides in the original cohort study. Inconvenience probably accounts for most of the non-participation—62.8% of refusers indicated only that they were not interested. In fact, refusers were more likely than participants to live at least 1 hour away from the testing centre (39% v 28%, respectively). However, it is possible that this resulted in the participation of healthier, more mobile people or, alternatively, less healthy people who were no longer working. Because most of the parkinsonism found was slight, it is unlikely to have played much part in a subject's willingness or ability to participate in this study. However, if exposure to pesticides were related to more severe parkinsonism that decreased participation due to consequent impairment or death, then we may have underestimated, or even missed, some associations. Notably, Bennett et al 34 found a twofold increase in risk of total mortality associated with parkinsonism. Incomplete follow up of the original Washington State cohort and limitations of death certificate data prevent us from adequately considering this issue.
Because of the many comparisons in this analysis, some of the associations found might have occurred by chance alone. The association between parkinsonism and long term use of pesticides was only marginally significant. However, a similarly increased, non-significant, risk was also found for medium term use of pesticides, although a trend test did not show a significant exposure-response relation. Also, increased, although non-significant, risks were associated with long term use of insecticides and herbicides.
Misclassification of parkinsonism is also of concern. Cases of parkinsonism in this study presented with only slight or mild signs. As the nurse who performed the neurological examination was blinded to the exposure of the subjects, difficulty in assessing the presence or absence of such subtle signs most likely resulted in non-differential misclassification. When the classification of parkinsonism was modified to account for possible overascertainment of rigidity, the magnitude of the association of parkinsonism with long term exposure to pesticides changed little, although the relation became non-significant. Excluding subjects reporting other conditions with possible parkinsonian features increased, or had little effect on, this association.
The low prevalence of parkinsonism among subjects with short term exposure to pesticides relative to subjects with no exposure or long term exposure raises the possibility that the associations found may be due to an abnormally low prevalence of parkinsonism among subjects with short term exposure. Arguing against this are the similarities in age specific prevalences of parkinsonism between subjects with short term exposure in this study and those in the Boston prevalence study.34 Use of a low exposure (internal) reference group (as well as a non-exposed (external) group) was intended to reduce the risk of bias by comparing groups likely to be most similar apart from exposure.31 Analysis of health conditions reported in the original study also showed no pattern of excess disease in any exposure group, indicating a lack of selection bias in the original study.
Our finding of increased risk of parkinsonism for general use of pesticides but not for specific pesticides may be due to increasing recall error as probing of historical exposures goes from general to specific. Such misclassification of exposure is likely to be non-differential for parkinsonism in this study, potentially masking certain associations. We tried to minimise this problem by providing subjects with a comprehensive list of pesticides they were likely to have used, including various common trade names for each pesticide, to facilitate recall and with a structured questionnaire that asked general questions before asking for specific information. Another possibility is that we did not consider the appropriate pesticides despite carefully selecting the most likely candidates.
In conclusion, parkinsonism may be associated with long term occupational exposure to pesticides, although we did not detect associations with specific pesticides. Follow up studies in which information on exposure to pesticides is collected around the time of use and updated periodically may help to resolve such ambiguity.
Answers to multiple choice questions onOccupational methaemoglobinaemia by SM Bradberryet al pages 611–616
(1) (a) true; (b) false; (c) true; (d) false; (e) true
(2) (a) false; (b) true; (c) true; (d) true; (e) false
(3) (a) true; (b) true; (c) false; (d) true; (e) false
(4) (a) true; (b) false; (c) true; (d) false; (e) false
(5) (a) false; (b) false; (c) false; (d) false; (e) false
Acknowledgments
We thank Mary Lou Thompson for statistical assistance and Phillip Swanson for insight and advice on the signs of parkinsonism in this cohort. We also thank Judith Shipley, who performed the neurological examinations. This work was supported by the National Institute for Environmental Health Sciences training grant ES07262, the National Institute for Occupational Safety and Health-supported PNASH grant U07/CCU012926–02, the United States Environmental Protection Agency Cooperative Agreement CR 822789–01–0, and by the Worker's Compensation Fund of the State of Washington, which is administered by the Washington State Department of Labor and Industries. This manuscript was reviewed by the National Health and Environmental Effects Research Laboratory, United States Environmental Protection Agency, and approved for publication. Approval does not signify that the contents necessarily reflect the views and policies of the Agency, nor does mention of trade names or commercial products constitute endorsement or recommendation.
References
Linked Articles
- Education