Abstract
Objectives
To measure eye blink frequency as a measure of trigeminal stimulation of the eye. Human subjects were exposed to oxidation mixtures representative of reactive indoor pollutants and clean air, from which relative changes in blink frequencies were measured.
Method
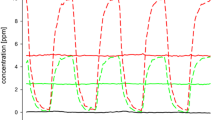
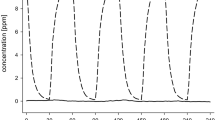
Male subjects (n=8) were exposed for 20 min to limonene oxidation products (LOPs), isoprene oxidation products (IOPs), nitrate radicals (NO3), their residual reactants, and clean air at 20% relative humidity. A baseline blink frequency was measured prior to and following each exposure (2×8 min). The subjects were exposed locally in the non-dominant eye and single blind in random order. Blinking was video-recorded and evaluated for full sessions of 36 min while the subjects viewed an educational film. The initial terpene concentrations were one to two orders of magnitude higher than mean indoor concentrations.
Results
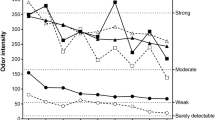
The mean blink frequency increased significantly during subjects’ exposure to gas-phase oxidation products at lower part-per-billion (ppb) levels of LOPs, 42% (P<0.0001) and NO3, 21% (P<0.022), compared with that at baseline. Neither the residual reactants nor clean air changed the blink frequency significantly. The findings coincided with qualitative reporting of weak eye irritation symptoms.
Conclusions
Changes in blink frequency appear to be a promising measure of trigeminal stimulation from exposure to eye irritants in ppb levels. Gaseous products of limonene and ozone, and reactive radicals may cause eye irritation indoors.



Similar content being viewed by others
References
Atkinson R (1986) Kinetics and mechanisms of the gas-phase reactions of the hydroxyl radical with organic compounds under atmospheric conditions. Chem Rev 86:169–201
Atkinson R (1991) Kinetics and mechanisms of the gas-phase reactions of the NO3 radical with organic compounds J Phys Chem Ref Data 20:459–507
Atkinson R, Aschmann SM, Arey J, Shorees B (1992) Formation of OH radicals in the gas phase reactions of O3 with a series of terpenes. J Geophys Res 97:6065–6073
Atkinson R, Baulch DL, Cox RA, Hampson RF Jr, Kerr JA, Rossi MJ, Troe J (1997) Evaluated kinetic and photochemical data for atmospheric chemistry: supplement VI. IUPAC subcommittee on gas kinetic data evaluation for atmospheric chemistry J Phys Chem Ref Data 26:1329–1499
Atkinson R, Baulch DL, Cox RA, Crowley JN, Hampson RF Jr, Kerr JA, Rossi MJ, Troe J (2001) Summary of evaluated kinetic and photochemical data for atmospheric chemistry. IUPAC Subcommittee on gas kinetic data evaluation for atmospheric chemistry. Web version, pp 1–56
Bluyssen PM, De Olivera Fernandes E, Groes L, Clausen G, Fanger PO, Valbjørn O, Bernhard CA, Roulet CA (1996) European indoor air quality audit project in 56 office buildings. Indoor Air 6:221–238
Bonn, B, Schuster G, Moortgat GK (2002) Influence of water vapour on the process of new particle formation during monoterpene ozonolysis, J Phys Chem A 106:2869–2881
Brightman HS, Moss N (2000). Sick building syndrome studies and the compilation of normative and comparative values. In: Spengler JD, Samet JM, McCarthy JF (eds) Indoor air quality handbook. McGraw-Hill, New York, pp 3.1–3.32
Craig JP (2002) Structure and function of the preocular tear film. The tear film: structure, function and clinical examination. Butterworth-Heinemann, Oxford, pp 18–50
DeMore WB, Golden DM, Hampson RF, Howard CJ, Kurylo MJ, Molina MJ. Ravishankara AR, Sander SP (1987) Chemical kinetics and photochemical data for use in stratospheric modeling evaluation, no 8. JPL Publication, pp 87–41
Doughty MJ, Fonn D, Richter D, Simpsom T, Caffery B, Gordon K (1997) A patient questionnaire approach to estimating the prevalence of dry eye symptoms in patients presenting to optometric practices across Canada. Optom Vis Sci 74:424–431
Emmen HH, Muijser H, Arts JH, Prinsen MK (2003) Human volunteer study with PGME: eye irritation during vapour exposure. Toxicol Lett 140–141:249–259
Fenske JD, Paulson SE (1999) Human breath emissions of VOCs. J Air Waste Manag Assoc 49:594–598
Grosjean E, Grosjean D (1996) Rate constants for the gas-phase reaction of ozone with 1,1-disubstituted alkenes. Int J Chem Kinet 28:911–918
Hauschildt P, Mølhave L, Kjaergaard SK (1999) Reactions of healthy persons and persons suffering from allergic rhinitis when exposed to office dust. Scand J Work Environ Health 25:442–449
Hodgson AT, Rudd AF, Beal D, Chandra S (2000) Volatile organic compound concentrations and emission rates in new manufactured and site-built houses. Indoor Air 10:178–192
Iregren A, Löf A, Toomingas A, Wang Z (1993) Irritation effects from experimental exposure to n-butyl acetate. Am J Ind Med 24:727–742
Junker MH, Danuser B, Monn C, Koller T (2001) Acute sensory responses of nonsmokers at very low environmental tobacco smoke concentrations in controlled laboratory settings. Environ Health Perspect 109:1045–1052
Kjaergaard SK, Hodgson M (2001) The assessment of irritation using clinical methods and questionnaires. AIHAJ 62:711–716
Kjaergaard SK, Mølhave L, Pedersen OF (1989) Human reactions to indoor air pollutants: n-decane. Environ Int 15:473–482
Kjaergaard SK, Mølhave L, Pedersen OF (1991) Human reactions to a mixture of indoor air volatile organic compounds. Atmos Environ 25A:1417–1426
Long CM, Suh HH, Koutrakis P (2000) Characterization of indoor particle sources using continuous mass and size monitors. J Air Waste Manag Assoc 50:1236–1250
Mendell MJ (1993) Non-specific symptoms in office workers: a review and summary of the epidemiologic literature. Indoor Air 3:227–236
Monster AW, Chan HC, O’Connor D (1978) Long-term trends in human eye blink rate. Biotel Patient Monit 5:206–222
Nihlén A, Löf A, Johanson G (1998a) Controlled ethyl tertiary-butyl ether (ETBE) exposure to male volunteers II. Acute effects. Toxicol Sci 46:143–150
Nihlén A, Wålinder R, Löf A, Johanson G (1998b) Experimental exposure to methyl tertiary-butyl ether. II. Acute effects in humans. Toxicol Appl Pharmacol 148:281–287
Norn MS (1992) Pollution keratoconjunctivitis. Acta Ophthalmol 70:269–273
Pan Z, Mølhave L, Kjaergaard SK (2000) Effects on eyes and nose in humans after experimental exposure to airborne office dust. Indoor Air 10:237–245
Paulson SE, Flagan RC, Seinfeld JH (1992) Atmospheric photooxidation of isoprene. Part I: the hydroxyl radical and ground state atomic oxygen reactions. Int J Chem Kinet 24:79–101
Prah JD, Hazucha M, Horstman D, Garlington R, Case M, Ashley D, Tepper J (1993) Pulmonary, respiratory, and irritant effects of exposure to a mixture of VOCs at three concentrations in young men. In: Jakkola JJK, Ilmarinen R, Seppänen O (eds) Proceedings of the 6th International Conference on Indoor Air Quality and Climate, vol 1, Indoor Air ‘93, Helsinki, pp 607–612
Saxena R, Srivastava S, Trivedi D, Anand E, Joshi S, Gupta SK (2003) Impact of environmental pollution on the eye. Acta Ophthalmol Scand 81:491–494
Seinfeld JH, Pandis SN (1998) Atmospheric chemistry and physics—from air pollution to climate change. Wiley, New York, chapt V
Shu Y, Atkinson R (1994) Rate constants for the gas-phase reactions of O3 with a series of terpenes and OH radical formation from the O3 reactions with sesquiterpenes at 296? 2 K. Int J Chem Kinet 26:1193–1205
Sibony PA, Evinger C (1998) Anatomy and physiology of normal and abnormal eyelid position and movement. In: Miller NR, Newman NJ (eds) Walsh & Hoyt’s clinical neuro-ophthalmology. Williams & Wilkins, Baltimore, pp 1509–1592
Versura P, Profazio V, Cellini M, Torreggiani A, Caramazza R (1999) Eye discomfort and air pollution. Ophthalmologica 213:103–109
Wahner A, Mentel TF, Sohn M (1998) Gas-phase reaction of N2O5 with water vapor: importance of heterogeneous hydrolysis of N2O5 and surface desorption of HNO3 in a large Teflon chamber. Geophys Res Lett 25:2169–2172
Walker JC, Kendall-Reed M, Utell MJ, Cain WS (2001) Human breathing and eye blink rate. Responses to airborne chemicals. Environ Health Perspect 109:507–512
Weber-Tschopp A, Fischer T, Grandjean E (1977) Reizwirkungen des formaldehyds (HCHO) auf den menschen. Int Arch Occup Environ Health 39:207–218
Weschler CJ (2000) Ozone in indoor environments: concentrations and chemistry. Indoor Air 10:269–288
Weschler CJ, Shields HC (2000) The influence of ventilation on reactions among indoor pollutants: modeling and experimental observations. Indoor Air 10:92–100
Weschler CJ, Shields HC, Naik DV (1994) Indoor chemistry involving O3, NO and NO2 as evidenced by 14 months of measurements at a site in Southern California. Environ Sci Technol 28:2120–2132
Wieslander G, Norbäck D, Lindgren T (2001) Experimental exposure to propylene glycol mist in aviation emergency training: acute ocular and respiratory effects. Occup Environ Med 58:649–655
Wilkins CK, Wolkoff P, Clausen PA, Hammer M, Nielsen GD (2003) Upper airway irritation of terpene/ozone oxidation products (TOPS). Dependence on reaction time, relative humidity and initial ozone concentration. Toxicol Lett 143:109–114
Wolkoff P (1998) Impact of air velocity, temperature, humidity, and air on long-term VOC emissions from building products. Atmos Environ 32:2659–2668
Wolkoff P, Nielsen GD (2001) Organic compounds in indoor air—their relevance for perceived indoor air quality. Atmos Environ 35:4407–4417
Wolkoff P, Clausen PA, Wilkins CK, Nielsen GD (2000) Formation of strong airway irritants in terpene/ozone mixtures. Indoor Air 10:82–91
Wolkoff P, Skov P, Franck C, Pedersen LN (2003) Eye irritation and environmental factors in the office environment. Hypotheses, causes, and a physiological model. Scand J Work Environ Health 29:411–430
Acknowledgements
We thank all subjects for participating in this study. Discussions with Dr. C.K. Wilkins are gratefully acknowledged, as is statistical assistance from Dr. Jacob Bue Bjørner and Karl Bang Christensen, Ph.D. We thank Dr. W.S. Cain for an eyepiece prototype.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Chemical reactions included in the modeling section and references to the rate constants are as follows:
Reaction | Rate constant | Reference |
|---|---|---|
(1) Isoprene + OH = products | 2.54×10−11 exp (3401/RT) | Atkinson 1986 |
(2) Isoprene + O3 = products | 5.6×10−15 exp (−15049/RT) | Grosjean and Grosjean 1996 |
(3) Isoprene + NO3= products | 3.02×10−12 exp (−3708/RT) | Atkinson 1991 |
(4) Isoprene + NO2 = products | 1.81×10−19 | Paulson et al. 1992 |
(5) NO2 + O3 = NO3 + O2 | 1.40 10−13 exp (−20537/RT) | Atkinson et al. 2001 |
(6) HO2 + O3 = OH + 2 O2 | 1.4×10−14 exp (−4989/RT) | Atkinson et al. 1997 |
(7) O3 + walla = 1.5 O2 (polyethylene) | 9.1 10−4 s−1 | This work |
(8) O3 + wall = 1.5 O2 (stainless steel) | 1.8 10−5 s−1 | This work |
(9) NO2 + OH = HNO3 | 7.51×10−11 (T/298 K)−0.60 | Atkinson et al. 1997 |
(10) NO2 + HO2 = HO2NO2 | 1.8×10−31 (T/298 K)−3.20 | Demore et al. 1987 |
(11) HO2NO2 = NO2 + HO2 | 5.7×1015 exp (−93122/RT) | Atkinson et al. 1997 |
(12) NO2 + NO3 = N2O5 | 2.01×10−12 (T/298 K)0.20 | Atkinson et al. 1997 |
(13) N2O5 = NO2 + NO3 | 9.71×1014 (T/298 K)0.10 exp(−92291/RT) | Atkinson et al. 1997 |
(14) HO2 + NO3 = OH + NO2 + O2 | 4 10−12 | Atkinson et al. 1997 |
(15) OH + NO3 = HO2 + NO2 | 2.01×10−11 | Atkinson et al. 1997 |
(16) HO2 + HO2 = O2 + H2O2 | 2.19×10−13 exp (4989/RT) | Atkinson et al. 1997 |
(17) N2O5 + H2O = 2 HNO3 | 2.51×10−22 | Wahner et al. 1998 |
(18) Limonene + O3 = 0.86 OH | 2.01×10−16 | Shu and Atkinson 1994 |
(19) Limonene + OH = products | 1.69×10−10 | Atkinson 1986 |
Rights and permissions
About this article
Cite this article
Klenø, J., Wolkoff, P. Changes in eye blink frequency as a measure of trigeminal stimulation by exposure to limonene oxidation products, isoprene oxidation products and nitrate radicals. Int Arch Occup Environ Health 77, 235–243 (2004). https://doi.org/10.1007/s00420-003-0502-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00420-003-0502-1