Abstract
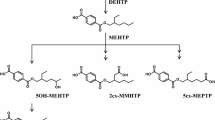
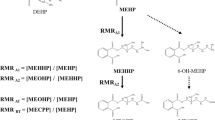
Metabolism of most diesters of phthalic acid in humans occurs by an initial phase I biotransformation in which phthalate monoesters are formed, followed by a phase II biotransformation in which phthalate monoesters react with glucuronic acid to form their respective glucuronide conjugates. The phase II conjugation increases water solubility and facilitates urinary excretion of phthalate, and reduces the potential biological activity because the putative biologically active species is the monoester metabolite. In this study, we report percentages of glucuronidation of four common phthalate monoesters, monoethyl (mEP), monobutyl (mBP), monobenzyl (mBzP), and mono-2-ethylhexyl phthalate (mEHP) in a subset of urine (mEP n=262, mBP n=283, mBzP n=328, mEHP n=119) and serum (mEP n=93, mBP n=149, mEHP n=141) samples from the general US population. The percentages of free and conjugated monoester excreted in urine differed for the various phthalates. For the more lipophilic monoesters (i.e., mBP, mBzP, and mEHP), the geometric mean of free monoester excretion ranged from 6 to 16%. The contrary was true for the most hydrophilic monoester, mEP, for which about 71% was excreted in urine as its free monoester. Furthermore, percentages of free and conjugated monoesters were similar for mEP, mBP and mEHP among serum and urine samples. Serum mBzP was largely below the method limit of detection. Interestingly, the serum mEP and mBP levels were less than 3% and 47%, respectively, of their urinary levels, whereas the level of mEHP was similar both in urine and serum.







Similar content being viewed by others
References
Albro PW, Moore B (1974) Identification of the metabolites of simple phthalate diesters in rat urine. J Chromatogr 94:209–218
Albro PW, Thomas RO (1973) Enzymatic hydrolysis of di-(2-ethylhexyl) phthalate by lipases. Biochim Biophys Acta Biochim 306:380–390
Albro PW, Thomas R, Fishbein L (1973) Metabolism of diethylhexyl phthalate by rats. Isolation and characterization of the urinary metabolites. J Chromatogr A, J 76:321–330
ATSDR (US Agency for Toxic Substances and Disease Registry) (1995) Toxicological profile for diethyl phthalate (DEP). US Department of Health and Human Services, Public Health Service, ATSDR, Atlanta GA. Available online via http://www.atsdr.cdc.gov/toxprofiles
ATSDR (US Agency for Toxic Substances and Disease Registry) (1997) Toxicological profile for di-n-octyl phthalate (DNOP). U.S. Department of Health and Human Services, Public Health Service, ATSDR, Atlanta GA. Available online via http://www.atsdr.cdc.gov/toxprofiles/tp95.html
ATSDR (US Agency for Toxic Substances and Disease Registry) (1999) Toxicological profile for di-n-butyl phthalate (DBP)... U.S. Department of Health and Human Services, Public Health Service, ATSDR, Atlanta GA. Available online via http://www.atsdr.cdc.gov/toxprofiles
ATSDR (US Agency for Toxic Substances and Disease Registry) (2000) Toxicological profile for di(2-ethylhexyl)phthalate (DEHP).. U.S. Department of Health and Human Services, Public Health Service, ATSDR, Atlanta GA. Available online via http://www.atsdr.cdc.gov/toxprofiles/
Autian J (1973) Toxicity and health threats of phthalate esters: review of the literature. Environ Health Perspect 4:3–26
Autian J (1982) Antifertility effects and dominant lethal assays for mutagenic effects of DEHP. Environ Health Perspect. 45:115–118
Banerjee S, Thuillier R, Culty M, Papadopoulos V, Brown TR, Banerjee PP (2002) In utero exposure to di(2-ethylhexyl) phthalate alters growth, tissue organization, and the expression of androgen receptor protein of rat prostate. Biol Reprod 66:254
Barr DB, Silva MJ, Kato K, Reidy JA, Malek NA, Hurtz D, Sadowski M, Needham LL, Calafat AM (2003) New directions in the quantitation of human exposure to phthalates. Environ Health Perspect Online, 24 February 2003, DOI 10.1289/ehp.6074
Blount BC, Milgram KE, Silva MJ, Malek NA, Reidy JA, Needham LL, Brock JW (2000a) Quantitative detection of eight phthalate metabolites in human urine using HPLC-APCI-MS/MS. Anal Chem 72:4127–4134
Blount BC, Silva MJ, Caudill SP, Needham LL, Pirkle JL, Sampson EJ, Lucier GW, Jackson RJ, Brock JW (2000b) Levels of seven urinary phthalate metabolites in a human reference population. Environ Health Perspect 108:979–982
Brock JW, Caudill SP, Silva MJ, Needham LL, Hilborn ED (2002) Phthalate monoesters levels in the urine of young children. Bull Environ Contamin Toxicol 68:309–314
CDC (Centers for Disease Control and Prevention) (2001) National report on human exposure to environmental chemicals 2001 (NCEH Pub. No. 01-0164). CDC National Center for Environmental Health, Division of Laboratory Sciences, Atlanta GA
CDC (Centers for Disease Control and Prevention) (2003) National report on human exposure to environmental chemicals 2003 (NCEH Pub. No. 01-0164). CDC, National Center for Environmental Health, Division of Laboratory Sciences, Atlanta GA
Douglas GR, Hugenholtz AP, Blakey DH (1986) Genetic toxicology of phthalate-esters—mutagenic and other genotoxic effects. Environ Health Perspect 65:255–262
Doull J, Cattley R, Elcombe C, Lake BG, Swenberg J, Wilkinson C, Williams G, van Gemert M (1999) A cancer risk assessment of di(2-ethylhexyl)phthalate: application of the new U.S. EPA Risk Assessment Guidelines. Toxicol Appl Pharmacol 29:327–357
Duty SM, Silva MJ, Barr DB, Brock JW, Ryan L, Chen Z, Herrick RF, Christiani D, Hauser R (2002) Urinary phthalate monoesters at general population exposure levels are associated with altered semen quality. Epidemiology 13:657
Heindel JJ, Powell CJ (1992) Phthalate ester effects on rat Sertoli-cell function-in vitro—effects of phthalate side-chain and age of animal. Toxicol Appl Pharmacol 115:116–123
Kato K, Silva MJ, Brock JW, Reidy JA, Malek NA, Hodge CC, Nakazawa H, Needham LL, Barr DB (2003) Quantitative detection of nine phthalate metabolites in human serum using reversed phase high performance liquid chromatography/electrospray ionization–tandem mass spectrometry. J Anal Toxicol (in press)
Mettang T, Thomas S, Kiefer T, Fischer FP, Kuhlmann U, Wodarz R, Rettenmeier AW (1996) Uraemic pruritus and exposure to di(2-ethylhexyl)phthalate (DEHP) in haemodialysis patients. Nephrol DialTransplant 11:2439–2443
Nassberger L, Arbin A, Ostelius J (1987) Exposure of patients to phthalates from polyvinyl-chloride tubes and bags during dialysis. Nephron 45:286–290
Silva MJ, Malek NA, Hodge CC, Reidy JA, Kato K, Barr DB, Needham LL, Brock JW (2003) Improved quantitative detection of 11 urinary phthalate metabolites in humans using liquid chromatography-atmospheric pressure chemical ionization–tandem mass spectrometry. J Chromatogr B 789/2:393–404
Acknowledgements
The authors acknowledge the National Center for Health Statistics for conducting NHANES. Use of trade names is for identification only and does not constitute endorsement by the US Department of Health and Human Services or the Centers for Disease Control and Prevention. The experiments comply with the current laws of the country in which the experiments were performed.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s00204-005-0653-9
Rights and permissions
About this article
Cite this article
Silva, M.J., Barr, D.B., Reidy, J.A. et al. Glucuronidation patterns of common urinary and serum monoester phthalate metabolites. Arch Toxicol 77, 561–567 (2003). https://doi.org/10.1007/s00204-003-0486-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-003-0486-3